Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- Science Education Policy Alliance
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items

Finding the formula of copper(II) oxide
In association with Nuffield Foundation
Use this class practical with your students to deduce the formula of copper(II) oxide from its reduction by methane
In this experiment, students heat copper(II) oxide in a glass tube while passing methane over it, reducing the copper(II) oxide to copper. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. This can also be used simply as an example of reduction.
The practical is likely to take up to an hour, perhaps more to analyse the results. Students who have not carried out this type of reaction before may find it helpful to have the techniques demonstrated first. It is not really suitable for a class practical for students under the age of 14, but could be a useful demonstration.
Each pair or group of students will need access to two gas taps.
The students will need access to matches or lighters to light their Bunsen burners. Alternatively, light a few around the room and students can light their own using a splint.
- Eye protection
- Reduction tube (hard glass test tube with small hole near closed end)
- 1-hole bung with glass tube to fit the reduction tube
- Rubber tubing
- Clamp stand, boss and clamp
- Bunsen burner
- Heat resistant mat
- Balance (must be accurate to at least 0.01 g)
- Copper(II) oxide (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), 2 spatulas
- Methane (natural gas) (EXTREMELY FLAMMABLE)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Copper(II) oxide, CuO(s), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC026 . For best results use analytical grade copper(II) oxide which has been dried by heating in an open dish at 300–400 °C for 10 min and then stored in a desiccator.
- Methane (natural gas), CH 4 (g), (EXTREMELY FLAMMABLE) – see CLEAPSS Hazcard HC045a . It is likely that most school will be using a class gas tap for this experiment. This gas forms explosive mixtures in the air.
- It is also worth referring to the CLEAPSS Laboratory Handbook Section 13.2.3 for further information about this experiment.
- Weigh the test tube with the bung in (mass 1). Put 2 spatulas of copper(II) oxide into the tube and spread it out as much as possible.
- Weigh the tube again, with the copper oxide in it (mass 2).
- Assemble the apparatus as shown in the diagram, but do not place the Bunsen burner underneath yet. Clamp the test tube as near to the bung as possible.
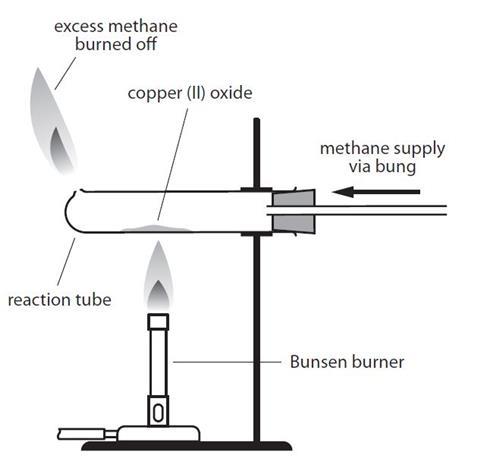
Source: Royal Society of Chemistry
The equipment required for reducing copper(II) oxide using methane
- Turn on the gas tap attached to the test tube about half way to get a steady flow of gas. This will pass methane through the apparatus.
- Wait for at least 10 seconds, to allow all the air to be flushed out of the tube and then light the gas coming out of the hole at the end of the tube. If this experiment is a student activity, a teacher should supervise this step. Take care not to lean over the tube as you light the gas. Adjust the gas tap so that the flame is about 3 cm high.
- Light the Bunsen burner and begin to heat the copper oxide in the tube. You will need to use a roaring flame (air hole fully open). You will need to pick up the Bunsen burner and move the flame around to heat every bit of the copper oxide. Ensure that the hottest part of the Bunsen burner flame (the top of the inner cone) is being used for heating. If there are parts which look unreacted, gently shake the tube – it will be very hot so do so by gently shaking the clamp stand.
- When all the copper oxide looks like it has reacted (it will look like copper), keep heating for a further minute or two and then turn off the Bunsen burner.
- Keep the methane passing over the product as it cools down to prevent it from reacting with any oxygen present and turning back into copper oxide. When the tube is cool, switch off the gas.
- Weigh the test tube with the bung and the product (mass 3).
Teaching notes
Students should have recorded the following masses:
- (mass 1) Test tube + bung
- (mass 2) Test tube + bung + copper oxide
- (mass 3) Test tube + bung + copper (product)
This should allow them to calculate the mass of the mass of the copper oxide (mass 2) – (mass 1) and the mass of the copper (mass 3) – (mass 1). They should also calculate the decrease in mass (mass 3) – (mass 2), which corresponds to the mass of oxygen.
With this information they can calculate the formula of the copper oxide.
Students will also need the relative atomic masses. Copper is 63.5 and oxygen is 16.
They should divide mass by the atomic mass for each element. This will give the number of moles of each.
Having done this for both elements, they should find the ratio between the two by dividing them both by the smallest number.
The ratio should be close to 1:1 as the formula of copper oxide is CuO.
Example calculation
Mass copper oxide = 1.76 g
Mass copper = 1.43 g
So, mass oxygen = 0.33 g
Number moles Cu = 1.43/63.5 = 0.02251
Number moles O = 0.33/16 = 0.020625
Divide by the smallest to give the ratio approx. 1 Cu: 1 O
This would suggest a formula of CuO, which is the correct formula.
Additional information
This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology .
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists .
© Nuffield Foundation and the Royal Society of Chemistry
- 14-16 years
- 16-18 years
- Practical experiments
- Demonstrations
- Redox chemistry
- Quantitative chemistry and stoichiometry
- Reactions and synthesis
Specification
- 2.2.1 recognise oxidation and reduction in terms of loss or gain of oxygen or hydrogen and identify in a reaction or symbol equation which species is oxidised and which is reduced (link to suitable industrial processes covered in this specification);
- 5 i. be able to use experimental data to calculate: empirical formulae. Calculations of empirical formula may involve composition by mass or percentage composition by mass data.
- Students should be able to: calculate empirical formula from data giving composition by mass or percentage by mass.
- c) calculations of empirical and molecular formulae, from composition by mass or percentage compositions by mass and relative molecular mass
- Some reactions may appear to involve a change in mass but this can usually be explained because a reactant or product is a gas and its mass has not been taken into account.
- Students should be able to balance an equation given the masses of reactants and products.
- Deduce the stoichiometry of an equation from the masses of reactants and products and explain the effect of a limiting quantity of a reactant.
- 1.46 Describe an experiment to determine the empirical formula of a simple compound such as magnesium oxide
- C3.4.7 use arithmetic computation and ratio when determining empirical formulae
- CM2.1.iv arithmetic computation and ratio when determining empirical formulae, balancing equations
- (p) how to calculate the formula of a compound from reacting mass data
- (d) how empirical and molecular formulae can be determined from given data
Related articles


Make worked examples count in quantitative chemistry
2024-12-11T06:36:00Z By Helen Skelton
Four teacher-tested approaches to encourage self-explanation and build learners’ confidence, engagement and understanding

Teaching conservation of mass at 14–16
2024-12-05T07:33:00Z By Matthew Parks
Help learners master the concept of conservation of mass with these hands-on teaching ideas

4 ways to teach the law of conservation of mass
2024-11-13T05:25:00Z By Kristy Turner
Improve 11–14 year-old learners’ understanding of this fundamental chemistry topic
2 readers' comments
Only registered users can comment on this article., more experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson Four out of five
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud
a) Reduction of copper (II) with zinc b) Oxidation of excess zinc with hydrochloric acid.

IMAGES
COMMENTS
Aug 11, 2011 · In the first reaction, copper metal is oxidized by nitric acid to form copper (II) nitrate, Cu(NO 3) 2. It is then converted to copper (II) hydroxide, Cu(OH) 2, by reaction with base. When this compound is heated, it is transformed to copper (II) oxide, CuO. Copper (II) oxide is then reacted with acid to form copper (II) sulfate, CuSO 4 ...
In this experiment, students heat copper(II) oxide in a glass tube while passing methane over it, reducing the copper(II) oxide to copper. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. This can also be used simply as an example of reduction.
the Oxide of Copper (II). We will do this by treating the Water soluble salt Copper (II) Sulfate Pentahydrate with Hydroxide Ion. This will generate Copper (II) Hydroxide, which, when heated strongly is converted to Copper (II) Oxide. As we have discussed, Copper forms an exception to the general Aufbau electron filling scheme in that
In this experiment you will observe a sequence of copper reactions. The sequence begins with copper metal and ends with copper metal, so it is called a cycle of copper reactions. Observations will be made for each reaction. Since no copper is added or removed between the initial and final reaction steps, copper can be quantitatively recovered.
EXPERIMENT Page 1 A Cycle of Copper Reactions PURPOSE To demonstrate a series of copper reactions: starting with copper metal, oxidizing the metal to put it into solution and then, form a copper hydroxide, an oxide, a sulfate, and lastly reducing the copper ion to produce copper metal again.
Copper Oxide • Copper(II) oxide is a basic oxide, so it dissolves in mineral acids such as hydrochloric acid, Sulfuric acid or nitric acid to give the corresponding copper(II) salts: CuO + 2 HNO3 → Cu(NO3)2 + H2O • It can also be reduced to copper metal using hydrogen or carbon monoxide: H2 + CuO → Cu + H2O • Copper (II) oxide has uses
In this case the copper oxide is reacted with sulfuric acid, thus the salt is the ionic compound copper (II) sulfate. CuO (s) + H 2 SO 4 (aq) ® CuSO 4 (aq) + H 2 O (l) Reaction 5. Reduction of copper (II) with zinc. In the final step, each copper (II) ion takes two electrons from a zinc atom, thereby regenerating the starting material ...
solid copper oxide. 26. If you still see black transfer the filter paper into the beaker. 27. Rinse the funnel with distiller water into the 250 mL beaker. 28. Once solid copper oxide is dissolved into copper chloride remove the filter. 29. Record your observations. 30. Write a balanced net ionic equation [On-Line Report Sheet Q14] and classify ...
Mar 4, 2022 · Two forms of copper oxide are found in nature, copper(I) oxide and copper(II) oxide. CuO copper (II) oxide Cu2O copper (I) oxide Copper oxides can be converted to metallic copper by reaction with molecular hydrogen or with carbon monoxide at high temperature: Lab #3: Empirical Formula of Copper Oxide
The sulphuric acid reacts with the solid copper oxide to dissolve the copper oxide from the filter paper and into the 250 mL Erlenmeyer beaker. This forms a light blue, translucent copper solution in the beaker. Indicating a colour change and change in transparency. Part E Observations (reforming Copper solid):