- Open access
- Published: 26 February 2019

Stem cells: past, present, and future
- Wojciech Zakrzewski 1 ,
- Maciej Dobrzyński 2 ,
- Maria Szymonowicz 1 &
- Zbigniew Rybak 1
Stem Cell Research & Therapy volume 10 , Article number: 68 ( 2019 ) Cite this article
614k Accesses
1070 Citations
54 Altmetric
Metrics details
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation. Quality control and teratoma formation assays are important procedures in assessing the properties of the stem cells tested. Derivation methods and the utilization of culturing media are crucial to set proper environmental conditions for controlled differentiation. Among many types of stem tissue applications, the use of graphene scaffolds and the potential of extracellular vesicle-based therapies require attention due to their versatility. The review is summarized by challenges that stem cell therapy must overcome to be accepted worldwide. A wide variety of possibilities makes this cutting edge therapy a turning point in modern medicine, providing hope for untreatable diseases.
Stem cell classification
Stem cells are unspecialized cells of the human body. They are able to differentiate into any cell of an organism and have the ability of self-renewal. Stem cells exist both in embryos and adult cells. There are several steps of specialization. Developmental potency is reduced with each step, which means that a unipotent stem cell is not able to differentiate into as many types of cells as a pluripotent one. This chapter will focus on stem cell classification to make it easier for the reader to comprehend the following chapters.
Totipotent stem cells are able to divide and differentiate into cells of the whole organism. Totipotency has the highest differentiation potential and allows cells to form both embryo and extra-embryonic structures. One example of a totipotent cell is a zygote, which is formed after a sperm fertilizes an egg. These cells can later develop either into any of the three germ layers or form a placenta. After approximately 4 days, the blastocyst’s inner cell mass becomes pluripotent. This structure is the source of pluripotent cells.
Pluripotent stem cells (PSCs) form cells of all germ layers but not extraembryonic structures, such as the placenta. Embryonic stem cells (ESCs) are an example. ESCs are derived from the inner cell mass of preimplantation embryos. Another example is induced pluripotent stem cells (iPSCs) derived from the epiblast layer of implanted embryos. Their pluripotency is a continuum, starting from completely pluripotent cells such as ESCs and iPSCs and ending on representatives with less potency—multi-, oligo- or unipotent cells. One of the methods to assess their activity and spectrum is the teratoma formation assay. iPSCs are artificially generated from somatic cells, and they function similarly to PSCs. Their culturing and utilization are very promising for present and future regenerative medicine.
Multipotent stem cells have a narrower spectrum of differentiation than PSCs, but they can specialize in discrete cells of specific cell lineages. One example is a haematopoietic stem cell, which can develop into several types of blood cells. After differentiation, a haematopoietic stem cell becomes an oligopotent cell. Its differentiation abilities are then restricted to cells of its lineage. However, some multipotent cells are capable of conversion into unrelated cell types, which suggests naming them pluripotent cells.
Oligopotent stem cells can differentiate into several cell types. A myeloid stem cell is an example that can divide into white blood cells but not red blood cells.
Unipotent stem cells are characterized by the narrowest differentiation capabilities and a special property of dividing repeatedly. Their latter feature makes them a promising candidate for therapeutic use in regenerative medicine. These cells are only able to form one cell type, e.g. dermatocytes.
Stem cell biology
A blastocyst is formed after the fusion of sperm and ovum fertilization. Its inner wall is lined with short-lived stem cells, namely, embryonic stem cells. Blastocysts are composed of two distinct cell types: the inner cell mass (ICM), which develops into epiblasts and induces the development of a foetus, and the trophectoderm (TE). Blastocysts are responsible for the regulation of the ICM microenvironment. The TE continues to develop and forms the extraembryonic support structures needed for the successful origin of the embryo, such as the placenta. As the TE begins to form a specialized support structure, the ICM cells remain undifferentiated, fully pluripotent and proliferative [ 1 ]. The pluripotency of stem cells allows them to form any cell of the organism. Human embryonic stem cells (hESCs) are derived from the ICM. During the process of embryogenesis, cells form aggregations called germ layers: endoderm, mesoderm and ectoderm (Fig. 1 ), each eventually giving rise to differentiated cells and tissues of the foetus and, later on, the adult organism [ 2 ]. After hESCs differentiate into one of the germ layers, they become multipotent stem cells, whose potency is limited to only the cells of the germ layer. This process is short in human development. After that, pluripotent stem cells occur all over the organism as undifferentiated cells, and their key abilities are proliferation by the formation of the next generation of stem cells and differentiation into specialized cells under certain physiological conditions.
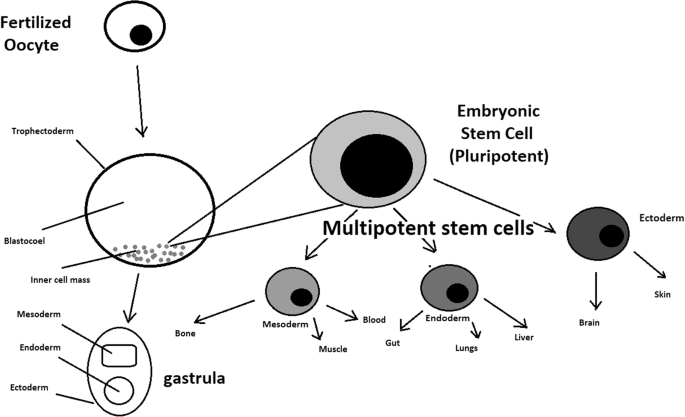
Oocyte development and formation of stem cells: the blastocoel, which is formed from oocytes, consists of embryonic stem cells that later differentiate into mesodermal, ectodermal, or endodermal cells. Blastocoel develops into the gastrula
Signals that influence the stem cell specialization process can be divided into external, such as physical contact between cells or chemical secretion by surrounding tissue, and internal, which are signals controlled by genes in DNA.
Stem cells also act as internal repair systems of the body. The replenishment and formation of new cells are unlimited as long as an organism is alive. Stem cell activity depends on the organ in which they are in; for example, in bone marrow, their division is constant, although in organs such as the pancreas, division only occurs under special physiological conditions.
Stem cell functional division
Whole-body development.
During division, the presence of different stem cells depends on organism development. Somatic stem cell ESCs can be distinguished. Although the derivation of ESCs without separation from the TE is possible, such a combination has growth limits. Because proliferating actions are limited, co-culture of these is usually avoided.
ESCs are derived from the inner cell mass of the blastocyst, which is a stage of pre-implantation embryo ca. 4 days after fertilization. After that, these cells are placed in a culture dish filled with culture medium. Passage is an inefficient but popular process of sub-culturing cells to other dishes. These cells can be described as pluripotent because they are able to eventually differentiate into every cell type in the organism. Since the beginning of their studies, there have been ethical restrictions connected to the medical use of ESCs in therapies. Most embryonic stem cells are developed from eggs that have been fertilized in an in vitro clinic, not from eggs fertilized in vivo.
Somatic or adult stem cells are undifferentiated and found among differentiated cells in the whole body after development. The function of these cells is to enable the healing, growth, and replacement of cells that are lost each day. These cells have a restricted range of differentiation options. Among many types, there are the following:
Mesenchymal stem cells are present in many tissues. In bone marrow, these cells differentiate mainly into the bone, cartilage, and fat cells. As stem cells, they are an exception because they act pluripotently and can specialize in the cells of any germ layer.
Neural cells give rise to nerve cells and their supporting cells—oligodendrocytes and astrocytes.
Haematopoietic stem cells form all kinds of blood cells: red, white, and platelets.
Skin stem cells form, for example, keratinocytes, which form a protective layer of skin.
The proliferation time of somatic stem cells is longer than that of ESCs. It is possible to reprogram adult stem cells back to their pluripotent state. This can be performed by transferring the adult nucleus into the cytoplasm of an oocyte or by fusion with the pluripotent cell. The same technique was used during cloning of the famous Dolly sheep.
hESCs are involved in whole-body development. They can differentiate into pluripotent, totipotent, multipotent, and unipotent cells (Fig. 2 ) [ 2 ].
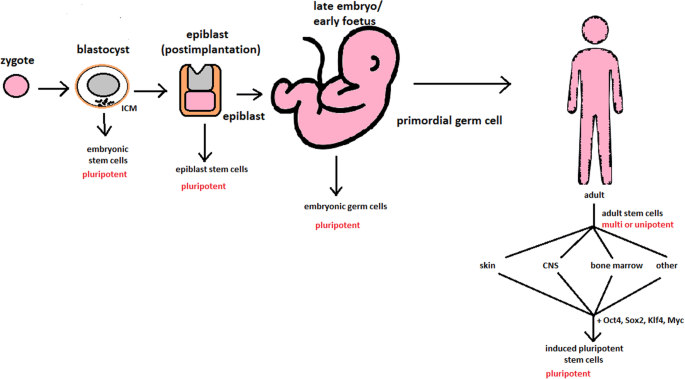
Changes in the potency of stem cells in human body development. Potency ranges from pluripotent cells of the blastocyst to unipotent cells of a specific tissue in a human body such as the skin, CNS, or bone marrow. Reversed pluripotency can be achieved by the formation of induced pluripotent stem cells using either octamer-binding transcription factor (Oct4), sex-determining region Y (Sox2), Kruppel-like factor 4 (Klf4), or the Myc gene
Pluripotent cells can be named totipotent if they can additionally form extraembryonic tissues of the embryo. Multipotent cells are restricted in differentiating to each cell type of given tissue. When tissue contains only one lineage of cells, stem cells that form them are called either called oligo- or unipotent.
iPSC quality control and recognition by morphological differences
The comparability of stem cell lines from different individuals is needed for iPSC lines to be used in therapeutics [ 3 ]. Among critical quality procedures, the following can be distinguished:
Short tandem repeat analysis—This is the comparison of specific loci on the DNA of the samples. It is used in measuring an exact number of repeating units. One unit consists of 2 to 13 nucleotides repeating many times on the DNA strand. A polymerase chain reaction is used to check the lengths of short tandem repeats. The genotyping procedure of source tissue, cells, and iPSC seed and master cell banks is recommended.
Identity analysis—The unintentional switching of lines, resulting in other stem cell line contamination, requires rigorous assay for cell line identification.
Residual vector testing—An appearance of reprogramming vectors integrated into the host genome is hazardous, and testing their presence is a mandatory procedure. It is a commonly used procedure for generating high-quality iPSC lines. An acceptable threshold in high-quality research-grade iPSC line collections is ≤ 1 plasmid copies per 100 cells. During the procedure, 2 different regions, common to all plasmids, should be used as specific targets, such as EBNA and CAG sequences [ 3 ]. To accurately represent the test reactions, a standard curve needs to be prepared in a carrier of gDNA from a well-characterized hPSC line. For calculations of plasmid copies per cell, it is crucial to incorporate internal reference gDNA sequences to allow the quantification of, for example, ribonuclease P (RNaseP) or human telomerase reverse transcriptase (hTERT).
Karyotype—A long-term culture of hESCs can accumulate culture-driven mutations [ 4 ]. Because of that, it is crucial to pay additional attention to genomic integrity. Karyotype tests can be performed by resuscitating representative aliquots and culturing them for 48–72 h before harvesting cells for karyotypic analysis. If abnormalities are found within the first 20 karyotypes, the analysis must be repeated on a fresh sample. When this situation is repeated, the line is evaluated as abnormal. Repeated abnormalities must be recorded. Although karyology is a crucial procedure in stem cell quality control, the single nucleotide polymorphism (SNP) array, discussed later, has approximately 50 times higher resolution.
Viral testing—When assessing the quality of stem cells, all tests for harmful human adventitious agents must be performed (e.g. hepatitis C or human immunodeficiency virus). This procedure must be performed in the case of non-xeno-free culture agents.
Bacteriology—Bacterial or fungal sterility tests can be divided into culture- or broth-based tests. All the procedures must be recommended by pharmacopoeia for the jurisdiction in which the work is performed.
Single nucleotide polymorphism arrays—This procedure is a type of DNA microarray that detects population polymorphisms by enabling the detection of subchromosomal changes and the copy-neutral loss of heterozygosity, as well as an indication of cellular transformation. The SNP assay consists of three components. The first is labelling fragmented nucleic acid sequences with fluorescent dyes. The second is an array that contains immobilized allele-specific oligonucleotide (ASO) probes. The last component detects, records, and eventually interprets the signal.
Flow cytometry—This is a technique that utilizes light to count and profile cells in a heterogeneous fluid mixture. It allows researchers to accurately and rapidly collect data from heterogeneous fluid mixtures with live cells. Cells are passed through a narrow channel one by one. During light illumination, sensors detect light emitted or refracted from the cells. The last step is data analysis, compilation and integration into a comprehensive picture of the sample.
Phenotypic pluripotency assays—Recognizing undifferentiated cells is crucial in successful stem cell therapy. Among other characteristics, stem cells appear to have a distinct morphology with a high nucleus to cytoplasm ratio and a prominent nucleolus. Cells appear to be flat with defined borders, in contrast to differentiating colonies, which appear as loosely located cells with rough borders [ 5 ]. It is important that images of ideal and poor quality colonies for each cell line are kept in laboratories, so whenever there is doubt about the quality of culture, it can always be checked according to the representative image. Embryoid body formation or directed differentiation of monolayer cultures to produce cell types representative of all three embryonic germ layers must be performed. It is important to note that colonies cultured under different conditions may have different morphologies [ 6 ].
Histone modification and DNA methylation—Quality control can be achieved by using epigenetic analysis tools such as histone modification or DNA methylation. When stem cells differentiate, the methylation process silences pluripotency genes, which reduces differentiation potential, although other genes may undergo demethylation to become expressed [ 7 ]. It is important to emphasize that stem cell identity, together with its morphological characteristics, is also related to its epigenetic profile [ 8 , 9 ]. According to Brindley [ 10 ], there is a relationship between epigenetic changes, pluripotency, and cell expansion conditions, which emphasizes that unmethylated regions appear to be serum-dependent.
hESC derivation and media
hESCs can be derived using a variety of methods, from classic culturing to laser-assisted methodologies or microsurgery [ 11 ]. hESC differentiation must be specified to avoid teratoma formation (see Fig. 3 ).
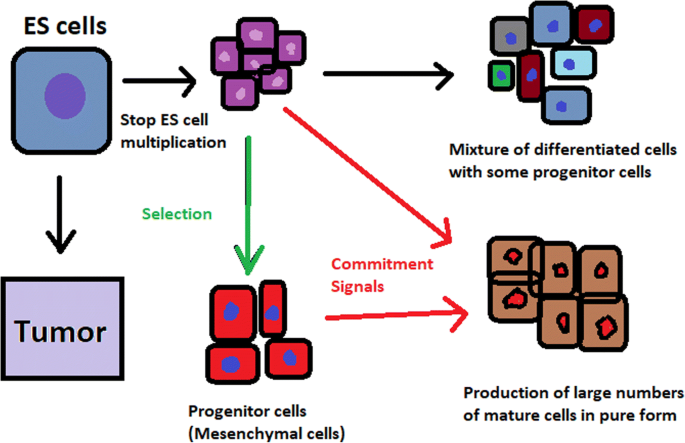
Spontaneous differentiation of hESCs causes the formation of a heterogeneous cell population. There is a different result, however, when commitment signals (in forms of soluble factors and culture conditions) are applied and enable the selection of progenitor cells
hESCs spontaneously differentiate into embryonic bodies (EBs) [ 12 ]. EBs can be studied instead of embryos or animals to predict their effects on early human development. There are many different methods for acquiring EBs, such as bioreactor culture [ 13 ], hanging drop culture [ 12 ], or microwell technology [ 14 , 15 ]. These methods allow specific precursors to form in vitro [ 16 ].
The essential part of these culturing procedures is a separation of inner cell mass to culture future hESCs (Fig. 4 ) [ 17 ]. Rosowski et al. [ 18 ] emphasizes that particular attention must be taken in controlling spontaneous differentiation. When the colony reaches the appropriate size, cells must be separated. The occurrence of pluripotent cells lasts for 1–2 days. Because the classical utilization of hESCs caused ethical concerns about gastrulas used during procedures, Chung et al. [ 19 ] found out that it is also possible to obtain hESCs from four cell embryos, leaving a higher probability of embryo survival. Additionally, Zhang et al. [ 20 ] used only in vitro fertilization growth-arrested cells.
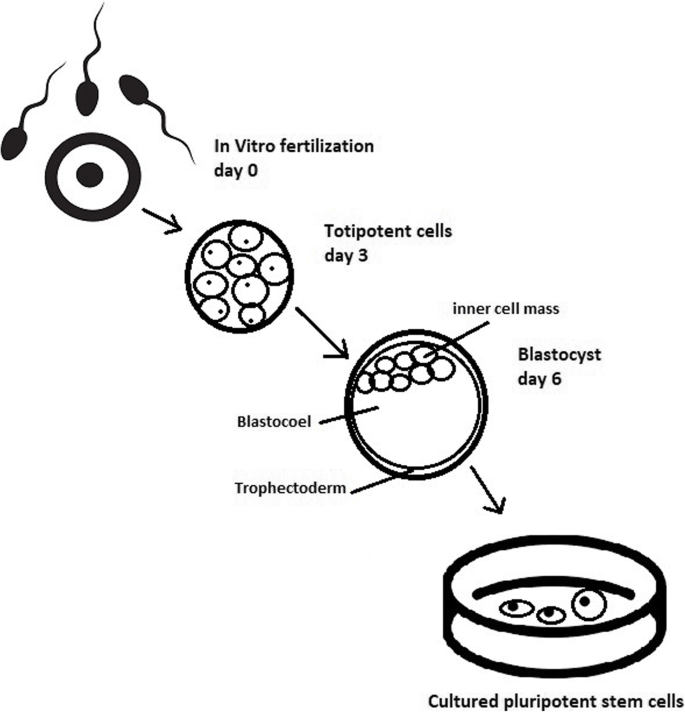
Culturing of pluripotent stem cells in vitro. Three days after fertilization, totipotent cells are formed. Blastocysts with ICM are formed on the sixth day after fertilization. Pluripotent stem cells from ICM can then be successfully transmitted on a dish
Cell passaging is used to form smaller clusters of cells on a new culture surface [ 21 ]. There are four important passaging procedures.
Enzymatic dissociation is a cutting action of enzymes on proteins and adhesion domains that bind the colony. It is a gentler method than the manual passage. It is crucial to not leave hESCs alone after passaging. Solitary cells are more sensitive and can easily undergo cell death; collagenase type IV is an example [ 22 , 23 ].
Manual passage , on the other hand, focuses on using cell scratchers. The selection of certain cells is not necessary. This should be done in the early stages of cell line derivation [ 24 ].
Trypsin utilization allows a healthy, automated hESC passage. Good Manufacturing Practice (GMP)-grade recombinant trypsin is widely available in this procedure [ 24 ]. However, there is a risk of decreasing the pluripotency and viability of stem cells [ 25 ]. Trypsin utilization can be halted with an inhibitor of the protein rho-associated protein kinase (ROCK) [ 26 ].
Ethylenediaminetetraacetic acid ( EDTA ) indirectly suppresses cell-to-cell connections by chelating divalent cations. Their suppression promotes cell dissociation [ 27 ].
Stem cells require a mixture of growth factors and nutrients to differentiate and develop. The medium should be changed each day.
Traditional culture methods used for hESCs are mouse embryonic fibroblasts (MEFs) as a feeder layer and bovine serum [ 28 ] as a medium. Martin et al. [ 29 ] demonstrated that hESCs cultured in the presence of animal products express the non-human sialic acid, N -glycolylneuraminic acid (NeuGc). Feeder layers prevent uncontrolled proliferation with factors such as leukaemia inhibitory factor (LIF) [ 30 ].
First feeder layer-free culture can be supplemented with serum replacement, combined with laminin [ 31 ]. This causes stable karyotypes of stem cells and pluripotency lasting for over a year.
Initial culturing media can be serum (e.g. foetal calf serum FCS), artificial replacement such as synthetic serum substitute (SSS), knockout serum replacement (KOSR), or StemPro [ 32 ]. The simplest culture medium contains only eight essential elements: DMEM/F12 medium, selenium, NaHCO 3, l -ascorbic acid, transferrin, insulin, TGFβ1, and FGF2 [ 33 ]. It is not yet fully known whether culture systems developed for hESCs can be allowed without adaptation in iPSC cultures.
Turning point in stem cell therapy
The turning point in stem cell therapy appeared in 2006, when scientists Shinya Yamanaka, together with Kazutoshi Takahashi, discovered that it is possible to reprogram multipotent adult stem cells to the pluripotent state. This process avoided endangering the foetus’ life in the process. Retrovirus-mediated transduction of mouse fibroblasts with four transcription factors (Oct-3/4, Sox2, KLF4, and c-Myc) [ 34 ] that are mainly expressed in embryonic stem cells could induce the fibroblasts to become pluripotent (Fig. 5 ) [ 35 ]. This new form of stem cells was named iPSCs. One year later, the experiment also succeeded with human cells [ 36 ]. After this success, the method opened a new field in stem cell research with a generation of iPSC lines that can be customized and biocompatible with the patient. Recently, studies have focused on reducing carcinogenesis and improving the conduction system.
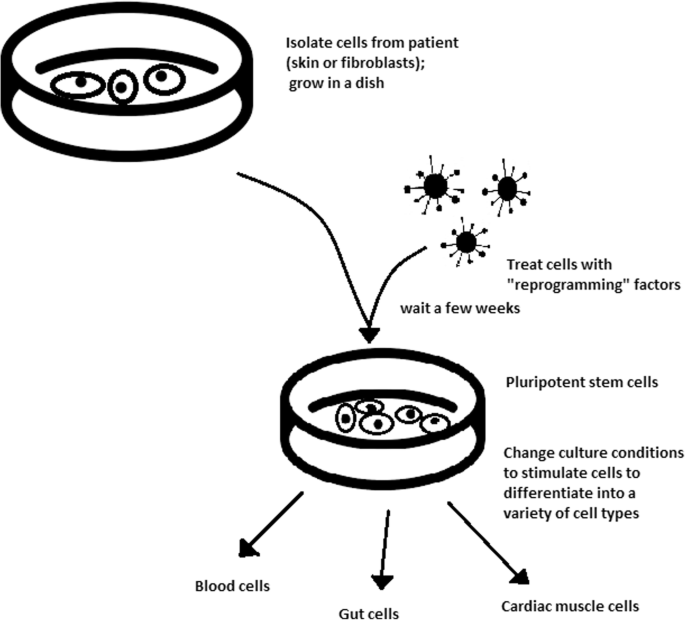
Retroviral-mediated transduction induces pluripotency in isolated patient somatic cells. Target cells lose their role as somatic cells and, once again, become pluripotent and can differentiate into any cell type of human body
The turning point was influenced by former discoveries that happened in 1962 and 1987.
The former discovery was about scientist John Gurdon successfully cloning frogs by transferring a nucleus from a frog’s somatic cells into an oocyte. This caused a complete reversion of somatic cell development [ 37 ]. The results of his experiment became an immense discovery since it was previously believed that cell differentiation is a one-way street only, but his experiment suggested the opposite and demonstrated that it is even possible for a somatic cell to again acquire pluripotency [ 38 ].
The latter was a discovery made by Davis R.L. that focused on fibroblast DNA subtraction. Three genes were found that originally appeared in myoblasts. The enforced expression of only one of the genes, named myogenic differentiation 1 (Myod1), caused the conversion of fibroblasts into myoblasts, showing that reprogramming cells is possible, and it can even be used to transform cells from one lineage to another [ 39 ].
Although pluripotency can occur naturally only in embryonic stem cells, it is possible to induce terminally differentiated cells to become pluripotent again. The process of direct reprogramming converts differentiated somatic cells into iPSC lines that can form all cell types of an organism. Reprogramming focuses on the expression of oncogenes such as Myc and Klf4 (Kruppel-like factor 4). This process is enhanced by a downregulation of genes promoting genome stability, such as p53. Additionally, cell reprogramming involves histone alteration. All these processes can cause potential mutagenic risk and later lead to an increased number of mutations. Quinlan et al. [ 40 ] checked fully pluripotent mouse iPSCs using whole genome DNA sequencing and structural variation (SV) detection algorithms. Based on those studies, it was confirmed that although there were single mutations in the non-genetic region, there were non-retrotransposon insertions. This led to the conclusion that current reprogramming methods can produce fully pluripotent iPSCs without severe genomic alterations.
During the course of development from pluripotent hESCs to differentiated somatic cells, crucial changes appear in the epigenetic structure of these cells. There is a restriction or permission of the transcription of genes relevant to each cell type. When somatic cells are being reprogrammed using transcription factors, all the epigenetic architecture has to be reconditioned to achieve iPSCs with pluripotency [ 41 ]. However, cells of each tissue undergo specific somatic genomic methylation. This influences transcription, which can further cause alterations in induced pluripotency [ 42 ].
Source of iPSCs
Because pluripotent cells can propagate indefinitely and differentiate into any kind of cell, they can be an unlimited source, either for replacing lost or diseased tissues. iPSCs bypass the need for embryos in stem cell therapy. Because they are made from the patient’s own cells, they are autologous and no longer generate any risk of immune rejection.
At first, fibroblasts were used as a source of iPSCs. Because a biopsy was needed to achieve these types of cells, the technique underwent further research. Researchers investigated whether more accessible cells could be used in the method. Further, other cells were used in the process: peripheral blood cells, keratinocytes, and renal epithelial cells found in urine. An alternative strategy to stem cell transplantation can be stimulating a patient’s endogenous stem cells to divide or differentiate, occurring naturally when skin wounds are healing. In 2008, pancreatic exocrine cells were shown to be reprogrammed to functional, insulin-producing beta cells [ 43 ].
The best stem cell source appears to be the fibroblasts, which is more tempting in the case of logistics since its stimulation can be fast and better controlled [ 44 ].
- Teratoma formation assay
The self-renewal and differentiation capabilities of iPSCs have gained significant interest and attention in regenerative medicine sciences. To study their abilities, a quality-control assay is needed, of which one of the most important is the teratoma formation assay. Teratomas are benign tumours. Teratomas are capable of rapid growth in vivo and are characteristic because of their ability to develop into tissues of all three germ layers simultaneously. Because of the high pluripotency of teratomas, this formation assay is considered an assessment of iPSC’s abilities [ 45 ].
Teratoma formation rate, for instance, was observed to be elevated in human iPSCs compared to that in hESCs [ 46 ]. This difference may be connected to different differentiation methods and cell origins. Most commonly, the teratoma assay involves an injection of examined iPSCs subcutaneously or under the testis or kidney capsule in mice, which are immune-deficient [ 47 ]. After injection, an immature but recognizable tissue can be observed, such as the kidney tubules, bone, cartilage, or neuroepithelium [ 30 ]. The injection site may have an impact on the efficiency of teratoma formation [ 48 ].
There are three groups of markers used in this assay to differentiate the cells of germ layers. For endodermal tissue, there is insulin/C-peptide and alpha-1 antitrypsin [ 49 ]. For the mesoderm, derivatives can be used, e.g. cartilage matrix protein for the bone and alcian blue for the cartilage. As ectodermal markers, class III B botulin or keratin can be used for keratinocytes.
Teratoma formation assays are considered the gold standard for demonstrating the pluripotency of human iPSCs, demonstrating their possibilities under physiological conditions. Due to their actual tissue formation, they could be used for the characterization of many cell lineages [ 50 ].
Directed differentiation
To be useful in therapy, stem cells must be converted into desired cell types as necessary or else the whole regenerative medicine process will be pointless. Differentiation of ESCs is crucial because undifferentiated ESCs can cause teratoma formation in vivo. Understanding and using signalling pathways for differentiation is an important method in successful regenerative medicine. In directed differentiation, it is likely to mimic signals that are received by cells when they undergo successive stages of development [ 51 ]. The extracellular microenvironment plays a significant role in controlling cell behaviour. By manipulating the culture conditions, it is possible to restrict specific differentiation pathways and generate cultures that are enriched in certain precursors in vitro. However, achieving a similar effect in vivo is challenging. It is crucial to develop culture conditions that will allow the promotion of homogenous and enhanced differentiation of ESCs into functional and desired tissues.
Regarding the self-renewal of embryonic stem cells, Hwang et al. [ 52 ] noted that the ideal culture method for hESC-based cell and tissue therapy would be a defined culture free of either the feeder layer or animal components. This is because cell and tissue therapy requires the maintenance of large quantities of undifferentiated hESCs, which does not make feeder cells suitable for such tasks.
Most directed differentiation protocols are formed to mimic the development of an inner cell mass during gastrulation. During this process, pluripotent stem cells differentiate into ectodermal, mesodermal, or endodermal progenitors. Mall molecules or growth factors induce the conversion of stem cells into appropriate progenitor cells, which will later give rise to the desired cell type. There is a variety of signal intensities and molecular families that may affect the establishment of germ layers in vivo, such as fibroblast growth factors (FGFs) [ 53 ]; the Wnt family [ 54 ] or superfamily of transforming growth factors—β(TGFβ); and bone morphogenic proteins (BMP) [ 55 ]. Each candidate factor must be tested on various concentrations and additionally applied to various durations because the precise concentrations and times during which developing cells in embryos are influenced during differentiation are unknown. For instance, molecular antagonists of endogenous BMP and Wnt signalling can be used for ESC formation of ectoderm [ 56 ]. However, transient Wnt and lower concentrations of the TGFβ family trigger mesodermal differentiation [ 57 ]. Regarding endoderm formation, a higher activin A concentration may be required [ 58 , 59 ].
There are numerous protocols about the methods of forming progenitors of cells of each of germ layers, such as cardiomyocytes [ 60 ], hepatocytes [ 61 ], renal cells [ 62 ], lung cells [ 63 , 64 ], motor neurons [ 65 ], intestinal cells [ 66 ], or chondrocytes [ 67 ].
Directed differentiation of either iPSCs or ESCs into, e.g. hepatocytes, could influence and develop the study of the molecular mechanisms in human liver development. In addition, it could also provide the possibility to form exogenous hepatocytes for drug toxicity testing [ 68 ].
Levels of concentration and duration of action with a specific signalling molecule can cause a variety of factors. Unfortunately, for now, a high cost of recombinant factors is likely to limit their use on a larger scale in medicine. The more promising technique focuses on the use of small molecules. These can be used for either activating or deactivating specific signalling pathways. They enhance reprogramming efficiency by creating cells that are compatible with the desired type of tissue. It is a cheaper and non-immunogenic method.
One of the successful examples of small-molecule cell therapies is antagonists and agonists of the Hedgehog pathway. They show to be very useful in motor neuron regeneration [ 69 ]. Endogenous small molecules with their function in embryonic development can also be used in in vitro methods to induce the differentiation of cells; for example, retinoic acid, which is responsible for patterning the nervous system in vivo [ 70 ], surprisingly induced retinal cell formation when the laboratory procedure involved hESCs [ 71 ].
The efficacy of differentiation factors depends on functional maturity, efficiency, and, finally, introducing produced cells to their in vivo equivalent. Topography, shear stress, and substrate rigidity are factors influencing the phenotype of future cells [ 72 ].
The control of biophysical and biochemical signals, the biophysical environment, and a proper guide of hESC differentiation are important factors in appropriately cultured stem cells.
Stem cell utilization and their manufacturing standards and culture systems
The European Medicines Agency and the Food and Drug Administration have set Good Manufacturing Practice (GMP) guidelines for safe and appropriate stem cell transplantation. In the past, protocols used for stem cell transplantation required animal-derived products [ 73 ].
The risk of introducing animal antigens or pathogens caused a restriction in their use. Due to such limitations, the technique required an obvious update [ 74 ]. Now, it is essential to use xeno-free equivalents when establishing cell lines that are derived from fresh embryos and cultured from human feeder cell lines [ 75 ]. In this method, it is crucial to replace any non-human materials with xeno-free equivalents [ 76 ].
NutriStem with LN-511, TeSR2 with human recombinant laminin (LN-511), and RegES with human foreskin fibroblasts (HFFs) are commonly used xeno-free culture systems [ 33 ]. There are many organizations and international initiatives, such as the National Stem Cell Bank, that provide stem cell lines for treatment or medical research [ 77 ].
Stem cell use in medicine
Stem cells have great potential to become one of the most important aspects of medicine. In addition to the fact that they play a large role in developing restorative medicine, their study reveals much information about the complex events that happen during human development.
The difference between a stem cell and a differentiated cell is reflected in the cells’ DNA. In the former cell, DNA is arranged loosely with working genes. When signals enter the cell and the differentiation process begins, genes that are no longer needed are shut down, but genes required for the specialized function will remain active. This process can be reversed, and it is known that such pluripotency can be achieved by interaction in gene sequences. Takahashi and Yamanaka [ 78 ] and Loh et al. [ 79 ] discovered that octamer-binding transcription factor 3 and 4 (Oct3/4), sex determining region Y (SRY)-box 2 and Nanog genes function as core transcription factors in maintaining pluripotency. Among them, Oct3/4 and Sox2 are essential for the generation of iPSCs.
Many serious medical conditions, such as birth defects or cancer, are caused by improper differentiation or cell division. Currently, several stem cell therapies are possible, among which are treatments for spinal cord injury, heart failure [ 80 ], retinal and macular degeneration [ 81 ], tendon ruptures, and diabetes type 1 [ 82 ]. Stem cell research can further help in better understanding stem cell physiology. This may result in finding new ways of treating currently incurable diseases.
Haematopoietic stem cell transplantation
Haematopoietic stem cells are important because they are by far the most thoroughly characterized tissue-specific stem cell; after all, they have been experimentally studied for more than 50 years. These stem cells appear to provide an accurate paradigm model system to study tissue-specific stem cells, and they have potential in regenerative medicine.
Multipotent haematopoietic stem cell (HSC) transplantation is currently the most popular stem cell therapy. Target cells are usually derived from the bone marrow, peripheral blood, or umbilical cord blood [ 83 ]. The procedure can be autologous (when the patient’s own cells are used), allogenic (when the stem cell comes from a donor), or syngeneic (from an identical twin). HSCs are responsible for the generation of all functional haematopoietic lineages in blood, including erythrocytes, leukocytes, and platelets. HSC transplantation solves problems that are caused by inappropriate functioning of the haematopoietic system, which includes diseases such as leukaemia and anaemia. However, when conventional sources of HSC are taken into consideration, there are some important limitations. First, there is a limited number of transplantable cells, and an efficient way of gathering them has not yet been found. There is also a problem with finding a fitting antigen-matched donor for transplantation, and viral contamination or any immunoreactions also cause a reduction in efficiency in conventional HSC transplantations. Haematopoietic transplantation should be reserved for patients with life-threatening diseases because it has a multifactorial character and can be a dangerous procedure. iPSC use is crucial in this procedure. The use of a patient’s own unspecialized somatic cells as stem cells provides the greatest immunological compatibility and significantly increases the success of the procedure.
Stem cells as a target for pharmacological testing
Stem cells can be used in new drug tests. Each experiment on living tissue can be performed safely on specific differentiated cells from pluripotent cells. If any undesirable effect appears, drug formulas can be changed until they reach a sufficient level of effectiveness. The drug can enter the pharmacological market without harming any live testers. However, to test the drugs properly, the conditions must be equal when comparing the effects of two drugs. To achieve this goal, researchers need to gain full control of the differentiation process to generate pure populations of differentiated cells.
Stem cells as an alternative for arthroplasty
One of the biggest fears of professional sportsmen is getting an injury, which most often signifies the end of their professional career. This applies especially to tendon injuries, which, due to current treatment options focusing either on conservative or surgical treatment, often do not provide acceptable outcomes. Problems with the tendons start with their regeneration capabilities. Instead of functionally regenerating after an injury, tendons merely heal by forming scar tissues that lack the functionality of healthy tissues. Factors that may cause this failed healing response include hypervascularization, deposition of calcific materials, pain, or swelling [ 84 ].
Additionally, in addition to problems with tendons, there is a high probability of acquiring a pathological condition of joints called osteoarthritis (OA) [ 85 ]. OA is common due to the avascular nature of articular cartilage and its low regenerative capabilities [ 86 ]. Although arthroplasty is currently a common procedure in treating OA, it is not ideal for younger patients because they can outlive the implant and will require several surgical procedures in the future. These are situations where stem cell therapy can help by stopping the onset of OA [ 87 ]. However, these procedures are not well developed, and the long-term maintenance of hyaline cartilage requires further research.
Osteonecrosis of the femoral hip (ONFH) is a refractory disease associated with the collapse of the femoral head and risk of hip arthroplasty in younger populations [ 88 ]. Although total hip arthroplasty (THA) is clinically successful, it is not ideal for young patients, mostly due to the limited lifetime of the prosthesis. An increasing number of clinical studies have evaluated the therapeutic effect of stem cells on ONFH. Most of the authors demonstrated positive outcomes, with reduced pain, improved function, or avoidance of THA [ 89 , 90 , 91 ].
Rejuvenation by cell programming
Ageing is a reversible epigenetic process. The first cell rejuvenation study was published in 2011 [ 92 ]. Cells from aged individuals have different transcriptional signatures, high levels of oxidative stress, dysfunctional mitochondria, and shorter telomeres than in young cells [ 93 ]. There is a hypothesis that when human or mouse adult somatic cells are reprogrammed to iPSCs, their epigenetic age is virtually reset to zero [ 94 ]. This was based on an epigenetic model, which explains that at the time of fertilization, all marks of parenteral ageing are erased from the zygote’s genome and its ageing clock is reset to zero [ 95 ].
In their study, Ocampo et al. [ 96 ] used Oct4, Sox2, Klf4, and C-myc genes (OSKM genes) and affected pancreas and skeletal muscle cells, which have poor regenerative capacity. Their procedure revealed that these genes can also be used for effective regenerative treatment [ 97 ]. The main challenge of their method was the need to employ an approach that does not use transgenic animals and does not require an indefinitely long application. The first clinical approach would be preventive, focused on stopping or slowing the ageing rate. Later, progressive rejuvenation of old individuals can be attempted. In the future, this method may raise some ethical issues, such as overpopulation, leading to lower availability of food and energy.
For now, it is important to learn how to implement cell reprogramming technology in non-transgenic elder animals and humans to erase marks of ageing without removing the epigenetic marks of cell identity.
Cell-based therapies
Stem cells can be induced to become a specific cell type that is required to repair damaged or destroyed tissues (Fig. 6 ). Currently, when the need for transplantable tissues and organs outweighs the possible supply, stem cells appear to be a perfect solution for the problem. The most common conditions that benefit from such therapy are macular degenerations [ 98 ], strokes [ 99 ], osteoarthritis [ 89 , 90 ], neurodegenerative diseases, and diabetes [ 100 ]. Due to this technique, it can become possible to generate healthy heart muscle cells and later transplant them to patients with heart disease.
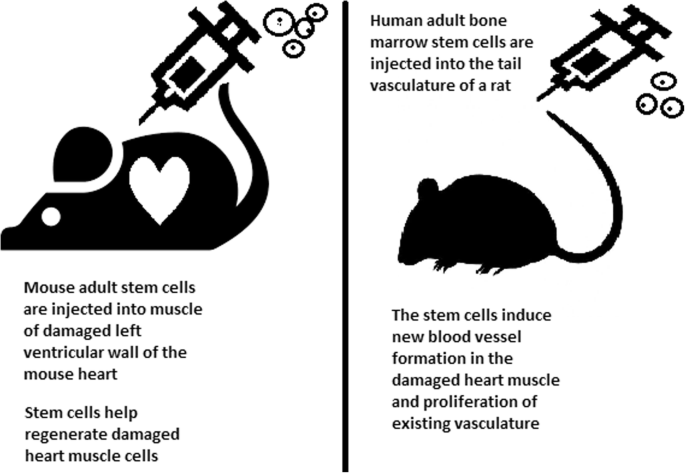
Stem cell experiments on animals. These experiments are one of the many procedures that proved stem cells to be a crucial factor in future regenerative medicine
In the case of type 1 diabetes, insulin-producing cells in the pancreas are destroyed due to an autoimmunological reaction. As an alternative to transplantation therapy, it can be possible to induce stem cells to differentiate into insulin-producing cells [ 101 ].
Stem cells and tissue banks
iPS cells with their theoretically unlimited propagation and differentiation abilities are attractive for the present and future sciences. They can be stored in a tissue bank to be an essential source of human tissue used for medical examination. The problem with conventional differentiated tissue cells held in the laboratory is that their propagation features diminish after time. This does not occur in iPSCs.
The umbilical cord is known to be rich in mesenchymal stem cells. Due to its cryopreservation immediately after birth, its stem cells can be successfully stored and used in therapies to prevent the future life-threatening diseases of a given patient.
Stem cells of human exfoliated deciduous teeth (SHED) found in exfoliated deciduous teeth has the ability to develop into more types of body tissues than other stem cells [ 102 ] (Table 1 ). Techniques of their collection, isolation, and storage are simple and non-invasive. Among the advantages of banking, SHED cells are:
Guaranteed donor-match autologous transplant that causes no immune reaction and rejection of cells [ 103 ]
Simple and painless for both child and parent
Less than one third of the cost of cord blood storage
Not subject to the same ethical concerns as embryonic stem cells [ 104 ]
In contrast to cord blood stem cells, SHED cells are able to regenerate into solid tissues such as connective, neural, dental, or bone tissue [ 105 , 106 ]
SHED can be useful for close relatives of the donor
Fertility diseases
In 2011, two researchers, Katsuhiko Hayashi et al. [ 107 ], showed in an experiment on mice that it is possible to form sperm from iPSCs. They succeeded in delivering healthy and fertile pups in infertile mice. The experiment was also successful for female mice, where iPSCs formed fully functional eggs .
Young adults at risk of losing their spermatogonial stem cells (SSC), mostly cancer patients, are the main target group that can benefit from testicular tissue cryopreservation and autotransplantation. Effective freezing methods for adult and pre-pubertal testicular tissue are available [ 108 ].
Qiuwan et al. [ 109 ] provided important evidence that human amniotic epithelial cell (hAEC) transplantation could effectively improve ovarian function by inhibiting cell apoptosis and reducing inflammation in injured ovarian tissue of mice, and it could be a promising strategy for the management of premature ovarian failure or insufficiency in female cancer survivors.
For now, reaching successful infertility treatments in humans appears to be only a matter of time, but there are several challenges to overcome. First, the process needs to have high efficiency; second, the chances of forming tumours instead of eggs or sperm must be maximally reduced. The last barrier is how to mature human sperm and eggs in the lab without transplanting them to in vivo conditions, which could cause either a tumour risk or an invasive procedure.
Therapy for incurable neurodegenerative diseases
Thanks to stem cell therapy, it is possible not only to delay the progression of incurable neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease (AD), and Huntington disease, but also, most importantly, to remove the source of the problem. In neuroscience, the discovery of neural stem cells (NSCs) has nullified the previous idea that adult CNS were not capable of neurogenesis [ 110 , 111 ]. Neural stem cells are capable of improving cognitive function in preclinical rodent models of AD [ 112 , 113 , 114 ]. Awe et al. [ 115 ] clinically derived relevant human iPSCs from skin punch biopsies to develop a neural stem cell-based approach for treating AD. Neuronal degeneration in Parkinson’s disease (PD) is focal, and dopaminergic neurons can be efficiently generated from hESCs. PD is an ideal disease for iPSC-based cell therapy [ 116 ]. However, this therapy is still in an experimental phase ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539501 /). Brain tissue from aborted foetuses was used on patients with Parkinson’s disease [ 117 ]. Although the results were not uniform, they showed that therapies with pure stem cells are an important and achievable therapy.
Stem cell use in dentistry
Teeth represent a very challenging material for regenerative medicine. They are difficult to recreate because of their function in aspects such as articulation, mastication, or aesthetics due to their complicated structure. Currently, there is a chance for stem cells to become more widely used than synthetic materials. Teeth have a large advantage of being the most natural and non-invasive source of stem cells.
For now, without the use of stem cells, the most common periodontological treatments are either growth factors, grafts, or surgery. For example, there are stem cells in periodontal ligament [ 118 , 119 ], which are capable of differentiating into osteoblasts or cementoblasts, and their functions were also assessed in neural cells [ 120 ]. Tissue engineering is a successful method for treating periodontal diseases. Stem cells of the root apical areas are able to recreate periodontal ligament. One of the possible methods of tissue engineering in periodontology is gene therapy performed using adenoviruses-containing growth factors [ 121 ].
As a result of animal studies, dentin regeneration is an effective process that results in the formation of dentin bridges [ 122 ].
Enamel is more difficult to regenerate than dentin. After the differentiation of ameloblastoma cells into the enamel, the former is destroyed, and reparation is impossible. Medical studies have succeeded in differentiating bone marrow stem cells into ameloblastoma [ 123 ].
Healthy dental tissue has a high amount of regular stem cells, although this number is reduced when tissue is either traumatized or inflamed [ 124 ]. There are several dental stem cell groups that can be isolated (Fig. 7 ).
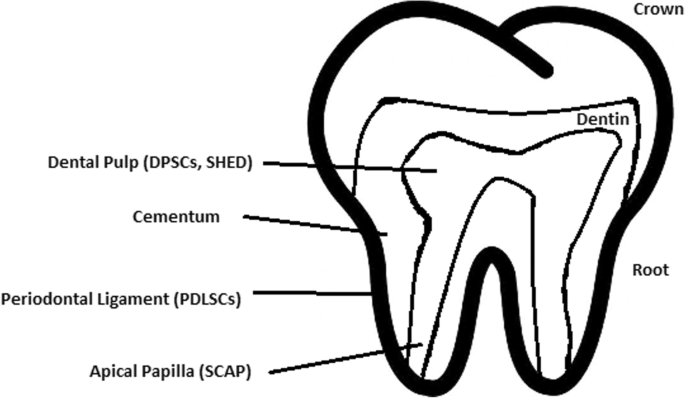
Localization of stem cells in dental tissues. Dental pulp stem cells (DPSCs) and human deciduous teeth stem cells (SHED) are located in the dental pulp. Periodontal ligaments stem cells are located in the periodontal ligament. Apical papilla consists of stem cells from the apical papilla (SCAP)
Dental pulp stem cell (DPSC)
These were the first dental stem cells isolated from the human dental pulp, which were [ 125 ] located inside dental pulp (Table 2 ). They have osteogenic and chondrogenic potential. Mesenchymal stem cells (MSCs) of the dental pulp, when isolated, appear highly clonogenic; they can be isolated from adult tissue (e.g. bone marrow, adipose tissue) and foetal (e.g. umbilical cord) [ 126 ] tissue, and they are able to differentiate densely [ 127 ]. MSCs differentiate into odontoblast-like cells and osteoblasts to form dentin and bone. Their best source locations are the third molars [ 125 ]. DPSCs are the most useful dental source of tissue engineering due to their easy surgical accessibility, cryopreservation possibility, increased production of dentin tissues compared to non-dental stem cells, and their anti-inflammatory abilities. These cells have the potential to be a source for maxillofacial and orthopaedic reconstructions or reconstructions even beyond the oral cavity. DPSCs are able to generate all structures of the developed tooth [ 128 ]. In particular, beneficial results in the use of DPSCs may be achieved when combined with other new therapies, such as periodontal tissue photobiomodulation (laser stimulation), which is an efficient technique in the stimulation of proliferation and differentiation into distinct cell types [ 129 ]. DPSCs can be induced to form neural cells to help treat neurological deficits.
Stem cells of human exfoliated deciduous teeth (SHED) have a faster rate of proliferation than DPSCs and differentiate into an even greater number of cells, e.g. other mesenchymal and non-mesenchymal stem cell derivatives, such as neural cells [ 130 ]. These cells possess one major disadvantage: they form a non-complete dentin/pulp-like complex in vivo. SHED do not undergo the same ethical concerns as embryonic stem cells. Both DPSCs and SHED are able to form bone-like tissues in vivo [ 131 ] and can be used for periodontal, dentin, or pulp regeneration. DPSCs and SHED can be used in treating, for example, neural deficits [ 132 ]. DPSCs alone were tested and successfully applied for alveolar bone and mandible reconstruction [ 133 ].
Periodontal ligament stem cells (PDLSCs)
These cells are used in periodontal ligament or cementum tissue regeneration. They can differentiate into mesenchymal cell lineages to produce collagen-forming cells, adipocytes, cementum tissue, Sharpey’s fibres, and osteoblast-like cells in vitro. PDLSCs exist both on the root and alveolar bone surfaces; however, on the latter, these cells have better differentiation abilities than on the former [ 134 ]. PDLSCs have become the first treatment for periodontal regeneration therapy because of their safety and efficiency [ 135 , 136 ].
Stem cells from apical papilla (SCAP)
These cells are mesenchymal structures located within immature roots. They are isolated from human immature permanent apical papilla. SCAP are the source of odontoblasts and cause apexogenesis. These stem cells can be induced in vitro to form odontoblast-like cells, neuron-like cells, or adipocytes. SCAP have a higher capacity of proliferation than DPSCs, which makes them a better choice for tissue regeneration [ 137 , 138 ].
Dental follicle stem cells (DFCs)
These cells are loose connective tissues surrounding the developing tooth germ. DFCs contain cells that can differentiate into cementoblasts, osteoblasts, and periodontal ligament cells [ 139 , 140 ]. Additionally, these cells proliferate after even more than 30 passages [ 141 ]. DFCs are most commonly extracted from the sac of a third molar. When DFCs are combined with a treated dentin matrix, they can form a root-like tissue with a pulp-dentin complex and eventually form tooth roots [ 141 ]. When DFC sheets are induced by Hertwig’s epithelial root sheath cells, they can produce periodontal tissue; thus, DFCs represent a very promising material for tooth regeneration [ 142 ].
Pulp regeneration in endodontics
Dental pulp stem cells can differentiate into odontoblasts. There are few methods that enable the regeneration of the pulp.
The first is an ex vivo method. Proper stem cells are grown on a scaffold before they are implanted into the root channel [ 143 ].
The second is an in vivo method. This method focuses on injecting stem cells into disinfected root channels after the opening of the in vivo apex. Additionally, the use of a scaffold is necessary to prevent the movement of cells towards other tissues. For now, only pulp-like structures have been created successfully.
Methods of placing stem cells into the root channel constitute are either soft scaffolding [ 144 ] or the application of stem cells in apexogenesis or apexification. Immature teeth are the best source [ 145 ]. Nerve and blood vessel network regeneration are extremely vital to keep pulp tissue healthy.
The potential of dental stem cells is mainly regarding the regeneration of damaged dentin and pulp or the repair of any perforations; in the future, it appears to be even possible to generate the whole tooth. Such an immense success would lead to the gradual replacement of implant treatments. Mandibulary and maxillary defects can be one of the most complicated dental problems for stem cells to address.
Acquiring non-dental tissue cells by dental stem cell differentiation
In 2013, it was reported that it is possible to grow teeth from stem cells obtained extra-orally, e.g. from urine [ 146 ]. Pluripotent stem cells derived from human urine were induced and generated tooth-like structures. The physical properties of the structures were similar to natural ones except for hardness [ 127 ]. Nonetheless, it appears to be a very promising technique because it is non-invasive and relatively low-cost, and somatic cells can be used instead of embryonic cells. More importantly, stem cells derived from urine did not form any tumours, and the use of autologous cells reduces the chances of rejection [ 147 ].
Use of graphene in stem cell therapy
Over recent years, graphene and its derivatives have been increasingly used as scaffold materials to mediate stem cell growth and differentiation [ 148 ]. Both graphene and graphene oxide (GO) represent high in-plane stiffness [ 149 ]. Because graphene has carbon and aromatic network, it works either covalently or non-covalently with biomolecules; in addition to its superior mechanical properties, graphene offers versatile chemistry. Graphene exhibits biocompatibility with cells and their proper adhesion. It also tested positively for enhancing the proliferation or differentiation of stem cells [ 148 ]. After positive experiments, graphene revealed great potential as a scaffold and guide for specific lineages of stem cell differentiation [ 150 ]. Graphene has been successfully used in the transplantation of hMSCs and their guided differentiation to specific cells. The acceleration skills of graphene differentiation and division were also investigated. It was discovered that graphene can serve as a platform with increased adhesion for both growth factors and differentiation chemicals. It was also discovered that π-π binding was responsible for increased adhesion and played a crucial role in inducing hMSC differentiation [ 150 ].
Therapeutic potential of extracellular vesicle-based therapies
Extracellular vesicles (EVs) can be released by virtually every cell of an organism, including stem cells [ 151 ], and are involved in intercellular communication through the delivery of their mRNAs, lipids, and proteins. As Oh et al. [ 152 ] prove, stem cells, together with their paracrine factors—exosomes—can become potential therapeutics in the treatment of, e.g. skin ageing. Exosomes are small membrane vesicles secreted by most cells (30–120 nm in diameter) [ 153 ]. When endosomes fuse with the plasma membrane, they become exosomes that have messenger RNAs (mRNAs) and microRNAs (miRNAs), some classes of non-coding RNAs (IncRNAs) and several proteins that originate from the host cell [ 154 ]. IncRNAs can bind to specific loci and create epigenetic regulators, which leads to the formation of epigenetic modifications in recipient cells. Because of this feature, exosomes are believed to be implicated in cell-to-cell communication and the progression of diseases such as cancer [ 155 ]. Recently, many studies have also shown the therapeutic use of exosomes derived from stem cells, e.g. skin damage and renal or lung injuries [ 156 ].
In skin ageing, the most important factor is exposure to UV light, called “photoageing” [ 157 ], which causes extrinsic skin damage, characterized by dryness, roughness, irregular pigmentation, lesions, and skin cancers. In intrinsic skin ageing, on the other hand, the loss of elasticity is a characteristic feature. The skin dermis consists of fibroblasts, which are responsible for the synthesis of crucial skin elements, such as procollagen or elastic fibres. These elements form either basic framework extracellular matrix constituents of the skin dermis or play a major role in tissue elasticity. Fibroblast efficiency and abundance decrease with ageing [ 158 ]. Stem cells can promote the proliferation of dermal fibroblasts by secreting cytokines such as platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), and basic fibroblast growth factor. Huh et al. [ 159 ] mentioned that a medium of human amniotic fluid-derived stem cells (hAFSC) positively affected skin regeneration after longwave UV-induced (UVA, 315–400 nm) photoageing by increasing the proliferation and migration of dermal fibroblasts. It was discovered that, in addition to the induction of fibroblast physiology, hAFSC transplantation also improved diseases in cases of renal pathology, various cancers, or stroke [ 160 , 161 ].
Oh [ 162 ] also presented another option for the treatment of skin wounds, either caused by physical damage or due to diabetic ulcers. Induced pluripotent stem cell-conditioned medium (iPSC-CM) without any animal-derived components induced dermal fibroblast proliferation and migration.
Natural cutaneous wound healing is divided into three steps: haemostasis/inflammation, proliferation, and remodelling. During the crucial step of proliferation, fibroblasts migrate and increase in number, indicating that it is a critical step in skin repair, and factors such as iPSC-CM that impact it can improve the whole cutaneous wound healing process. Paracrine actions performed by iPSCs are also important for this therapeutic effect [ 163 ]. These actions result in the secretion of cytokines such as TGF-β, interleukin (IL)-6, IL-8, monocyte chemotactic protein-1 (MCP-1), vascular endothelial growth factor (VEGF), platelet-derived growth factor-AA (PDGF-AA), and basic fibroblast growth factor (bFGF). Bae et al. [ 164 ] mentioned that TGF-β induced the migration of keratinocytes. It was also demonstrated that iPSC factors can enhance skin wound healing in vivo and in vitro when Zhou et al. [ 165 ] enhanced wound healing, even after carbon dioxide laser resurfacing in an in vivo study.
Peng et al. [ 166 ] investigated the effects of EVs derived from hESCs on in vitro cultured retinal glial, progenitor Müller cells, which are known to differentiate into retinal neurons. EVs appear heterogeneous in size and can be internalized by cultured Müller cells, and their proteins are involved in the induction and maintenance of stem cell pluripotency. These stem cell-derived vesicles were responsible for the neuronal trans-differentiation of cultured Müller cells exposed to them. However, the research article points out that the procedure was accomplished only on in vitro acquired retina.
Challenges concerning stem cell therapy
Although stem cells appear to be an ideal solution for medicine, there are still many obstacles that need to be overcome in the future. One of the first problems is ethical concern.
The most common pluripotent stem cells are ESCs. Therapies concerning their use at the beginning were, and still are, the source of ethical conflicts. The reason behind it started when, in 1998, scientists discovered the possibility of removing ESCs from human embryos. Stem cell therapy appeared to be very effective in treating many, even previously incurable, diseases. The problem was that when scientists isolated ESCs in the lab, the embryo, which had potential for becoming a human, was destroyed (Fig. 8 ). Because of this, scientists, seeing a large potential in this treatment method, focused their efforts on making it possible to isolate stem cells without endangering their source—the embryo.
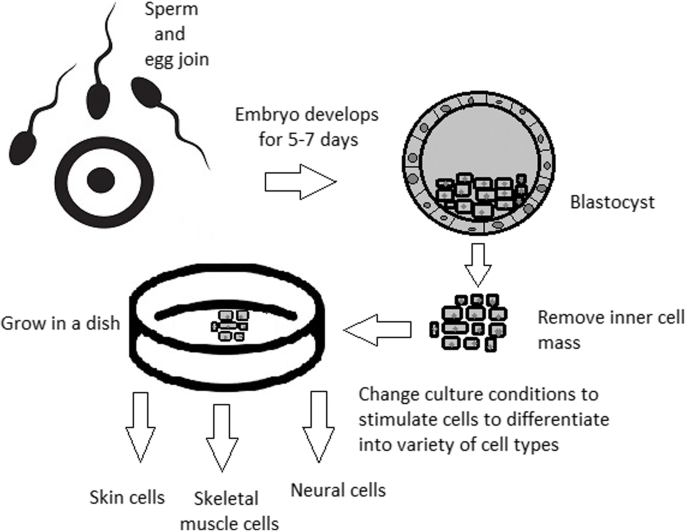
Use of inner cell mass pluripotent stem cells and their stimulation to differentiate into desired cell types
For now, while hESCs still remain an ethically debatable source of cells, they are potentially powerful tools to be used for therapeutic applications of tissue regeneration. Because of the complexity of stem cell control systems, there is still much to be learned through observations in vitro. For stem cells to become a popular and widely accessible procedure, tumour risk must be assessed. The second problem is to achieve successful immunological tolerance between stem cells and the patient’s body. For now, one of the best ideas is to use the patient’s own cells and devolve them into their pluripotent stage of development.
New cells need to have the ability to fully replace lost or malfunctioning natural cells. Additionally, there is a concern about the possibility of obtaining stem cells without the risk of morbidity or pain for either the patient or the donor. Uncontrolled proliferation and differentiation of cells after implementation must also be assessed before its use in a wide variety of regenerative procedures on living patients [ 167 ].
One of the arguments that limit the use of iPSCs is their infamous role in tumourigenicity. There is a risk that the expression of oncogenes may increase when cells are being reprogrammed. In 2008, a technique was discovered that allowed scientists to remove oncogenes after a cell achieved pluripotency, although it is not efficient yet and takes a longer amount of time. The process of reprogramming may be enhanced by deletion of the tumour suppressor gene p53, but this gene also acts as a key regulator of cancer, which makes it impossible to remove in order to avoid more mutations in the reprogrammed cell. The low efficiency of the process is another problem, which is progressively becoming reduced with each year. At first, the rate of somatic cell reprogramming in Yamanaka’s study was up to 0.1%. The use of transcription factors creates a risk of genomic insertion and further mutation of the target cell genome. For now, the only ethically acceptable operation is an injection of hESCs into mouse embryos in the case of pluripotency evaluation [ 168 ].
Stem cell obstacles in the future
Pioneering scientific and medical advances always have to be carefully policed in order to make sure they are both ethical and safe. Because stem cell therapy already has a large impact on many aspects of life, it should not be treated differently.
Currently, there are several challenges concerning stem cells. First, the most important one is about fully understanding the mechanism by which stem cells function first in animal models. This step cannot be avoided. For the widespread, global acceptance of the procedure, fear of the unknown is the greatest challenge to overcome.
The efficiency of stem cell-directed differentiation must be improved to make stem cells more reliable and trustworthy for a regular patient. The scale of the procedure is another challenge. Future stem cell therapies may be a significant obstacle. Transplanting new, fully functional organs made by stem cell therapy would require the creation of millions of working and biologically accurate cooperating cells. Bringing such complicated procedures into general, widespread regenerative medicine will require interdisciplinary and international collaboration.
The identification and proper isolation of stem cells from a patient’s tissues is another challenge. Immunological rejection is a major barrier to successful stem cell transplantation. With certain types of stem cells and procedures, the immune system may recognize transplanted cells as foreign bodies, triggering an immune reaction resulting in transplant or cell rejection.
One of the ideas that can make stem cells a “failsafe” is about implementing a self-destruct option if they become dangerous. Further development and versatility of stem cells may cause reduction of treatment costs for people suffering from currently incurable diseases. When facing certain organ failure, instead of undergoing extraordinarily expensive drug treatment, the patient would be able to utilize stem cell therapy. The effect of a successful operation would be immediate, and the patient would avoid chronic pharmacological treatment and its inevitable side effects.
Although these challenges facing stem cell science can be overwhelming, the field is making great advances each day. Stem cell therapy is already available for treating several diseases and conditions. Their impact on future medicine appears to be significant.
After several decades of experiments, stem cell therapy is becoming a magnificent game changer for medicine. With each experiment, the capabilities of stem cells are growing, although there are still many obstacles to overcome. Regardless, the influence of stem cells in regenerative medicine and transplantology is immense. Currently, untreatable neurodegenerative diseases have the possibility of becoming treatable with stem cell therapy. Induced pluripotency enables the use of a patient’s own cells. Tissue banks are becoming increasingly popular, as they gather cells that are the source of regenerative medicine in a struggle against present and future diseases. With stem cell therapy and all its regenerative benefits, we are better able to prolong human life than at any time in history.
Abbreviations
Basic fibroblast growth factor
Bone morphogenic proteins
Dental follicle stem cells
Dental pulp stem cells
Embryonic bodies
Embryonic stem cells
Fibroblast growth factors
Good Manufacturing Practice
Graphene oxide
Human amniotic fluid-derived stem cells
Human embryonic stem cells
Human foreskin fibroblasts
Inner cell mass
Non-coding RNA
Induced pluripotent stem cells
In vitro fertilization
Knockout serum replacement
Leukaemia inhibitory factor
Monocyte chemotactic protein-1
Fibroblasts
Messenger RNA
Mesenchymal stem cells of dental pulp
Myogenic differentiation
Osteoarthritis
Octamer-binding transcription factor 3 and 4
Platelet-derived growth factor
Platelet-derived growth factor-AA
Periodontal ligament stem cells
Rho-associated protein kinase
Stem cells from apical papilla
Stem cells of human exfoliated deciduous teeth
Synthetic Serum Substitute
Trophectoderm
Vascular endothelial growth factor
Transforming growth factors
Sukoyan MA, Vatolin SY, et al. Embryonic stem cells derived from morulae, inner cell mass, and blastocysts of mink: comparisons of their pluripotencies. Embryo Dev. 1993;36(2):148–58
Larijani B, Esfahani EN, Amini P, Nikbin B, Alimoghaddam K, Amiri S, Malekzadeh R, Yazdi NM, Ghodsi M, Dowlati Y, Sahraian MA, Ghavamzadeh A. Stem cell therapy in treatment of different diseases. Acta Medica Iranica. 2012:79–96 https://www.ncbi.nlm.nih.gov/pubmed/22359076 .
Sullivan S, Stacey GN, Akazawa C, et al. Quality guidelines for clinical-grade human induced pluripotent stem cell lines. Regenerative Med. 2018; https://doi.org/10.2217/rme-2018-0095 .
Amps K, Andrews PW, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 2011; 29 (12):1121–44.
Google Scholar
Amit M, Itskovitz-Eldor J. Atlas of human pluripotent stem cells: derivation and culturing. New York: Humana Press; 2012.
Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–46.
CAS PubMed Google Scholar
Kang MI. Transitional CpG methylation between promoters and retroelements of tissue-specific genes during human mesenchymal cell differentiation. J. Cell Biochem. 2007;102:224–39.
Vaes B, Craeye D, Pinxteren J. Quality control during manufacture of a stem cell therapeutic. BioProcess Int. 2012;10:50–5.
Bloushtain-Qimron N. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle. 2009;8:809–17.
Brindley DA. Peak serum: implications of serum supply for cell therapy manufacturing. Regenerative Medicine. 2012;7:809–17.
Solter D, Knowles BB. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975;72:5099–102.
CAS PubMed PubMed Central Google Scholar
Hoepfl G, Gassmann M, Desbaillets I. Differentiating embryonic stem cells into embryoid bodies. Methods Mole Biol. 2004;254:79–98 https://doi.org/10.1385/1-59259-741-6:079 .
Lim WF, Inoue-Yokoo T, Tan KS, Lai MI, Sugiyama D. Hematopoietic cell differentiation from embryonic and induced pluripotent stem cells. Stem Cell Res Ther. 2013;4(3):71. https://doi.org/10.1186/scrt222 .
Article CAS PubMed PubMed Central Google Scholar
Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27(36):6032–42. https://doi.org/10.1016/j.biomaterials.2006.07.012 .
Article CAS PubMed Google Scholar
Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of the visceral yolk sac, blood islands, and myocardium. J Embryol Exp Morphol. 1985;87:27–45.
Kurosawa HY. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–98.
Heins N, Englund MC, Sjoblom C, Dahl U, Tonning A, Bergh C, Lindahl A, Hanson C, Semb H. Derivation, characterization, and differentiation of human embryonic stem cells. Stem Cells. 2004;22:367–76.
Rosowski KA, Mertz AF, Norcross S, Dufresne ER, Horsley V. Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential. Sci Rep. 2015;5:Article number:14218.
PubMed Google Scholar
Chung Y, Klimanskaya I, Becker S, Li T, Maserati M, Lu SJ, Zdravkovic T, Ilic D, Genbacev O, Fisher S, Krtolica A, Lanza R. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2:113–7.
Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M, Stojkovic M. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669–76.
Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc. 2012;7:2029–40.
Ellerström C, Hyllner J, Strehl R. single cell enzymatic dissociation of human embryonic stem cells: a straightforward, robust, and standardized culture method. In: Turksen K, editor. Human embryonic stem cell protocols. Methods in molecular biology: Humana Press; 2009. p. 584.
Heng BC, Liu H, Ge Z, Cao T. Mechanical dissociation of human embryonic stem cell colonies by manual scraping after collagenase treatment is much more detrimental to cellular viability than is trypsinization with gentle pipetting. Biotechnol Appl Biochem. 2010;47(1):33–7.
Ellerstrom C, Strehl R, Noaksson K, Hyllner J, Semb H. Facilitated expansion of human embryonic stem cells by single-cell enzymatic dissociation. Stem Cells. 2007;25:1690–6.
Brimble SN, Zeng X, Weiler DA, Luo Y, Liu Y, Lyons IG, Freed WJ, Robins AJ, Rao MS, Schulz TC. Karyotypic stability, genotyping, differentiation, feeder-free maintenance, and gene expression sampling in three human embryonic stem cell lines deri. Stem Cells Dev. 2004;13:585–97.
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6.
Nie Y, Walsh P, Clarke DL, Rowley JA, Fellner T. Scalable passaging of adherent human pluripotent stem cells. 2014. https://doi.org/10.1371/journal.pone.0088012 .
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cellsexpress an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–32.
Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–90. https://doi.org/10.1038/336688a0 .
Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. 2001;19:971–4. https://doi.org/10.1038/nbt1001-971 .
Article CAS Google Scholar
Weathersbee PS, Pool TB, Ord T. Synthetic serum substitute (SSS): a globulin-enriched protein supplement for human embryo culture. J. Assist Reprod Genet. 1995;12:354–60.
Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–9.
Sommer CA, Mostoslavsky G. Experimental approaches for the generation of induced pluripotent stem cells. Stem Cell Res Ther. 2010;1:26.
PubMed PubMed Central Google Scholar
Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140(12):2457–61 https://doi.org/10.1242/dev.092551 .
Shi D, Lu F, Wei Y, et al. Buffalos ( Bubalus bubalis ) cloned by nuclear transfer of somatic cells. Biol. Reprod. 2007;77:285–91. https://doi.org/10.1095/biolreprod.107.060210 .
Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Development. 1962;10:622–40 http://dev.biologists.org/content/10/4/622 .
CAS Google Scholar
Kain K. The birth of cloning: an interview with John Gurdon. Dis Model Mech. 2009;2(1–2):9–10. https://doi.org/10.1242/dmm.002014 .
Article PubMed Central Google Scholar
Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;24(51(6)):987–1000.
Quinlan AR, Boland MJ, Leibowitz ML, et al. Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell. 2011;9(4):366–73.
Maherali N, Sridharan R, Xie W, Utika LJ, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70.
Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, Yu J, Hebrok M, Hochedlinger K, Costello JF, Song JS, Ramalho-Santos M. Incomplete DNA methylation underlines a transcriptional memory of somatic cells in human IPS cells. Nat Cell Biol. 2011;13:541–9.
Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32 https://doi.org/10.1038/nature07314 .
Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396:489–97.
Zhang Wendy, Y., de Almeida Patricia, E., and Wu Joseph, C. Teratoma formation: a tool for monitoring pluripotency in stem cell research. StemBook, ed. The Stem Cell Research Community . June 12, 2012. https://doi.org/10.3824/stembook.1.53.1 .
Narsinh KH, Sun N, Sanchez-Freire V, et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121(3):1217–21.
Gertow K, Przyborski S, Loring JF, Auerbach JM, Epifano O, Otonkoski T, Damjanov I, AhrlundRichter L. Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol . 2007, Chapter 1, Unit 1B 4. 3: 1B.4.1-1B.4.29.
Cooke MJ, Stojkovic M, Przyborski SA. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006;15(2):254–9.
Przyborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells. 2005;23:1242–50.
Tannenbaum SE, Turetsky TT, Singer O, Aizenman E, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, Perlman TS, Geva N, Levy O, Arbell D, Simon A, Ben-Meir A, Shufaro Y, Laufer N, Reubinoff BE. Derivation of xeno-free and GMP-grade human embryonic stem cells- platforms for future clinical applications. PLoS One. 2012;7:e35325.
Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–52.
Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2007;60(2):199–214. https://doi.org/10.1016/j.addr.2007.08.036 .
Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29.
Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–806.
Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–714.
Efthymiou AG, Chen G, Rao M, Chen G, Boehm M. Self-renewal and cell lineage differentiation strategies in human embryonic stem cells and induced pluripotent stem cells. Expert Opin Biol Ther. 2014;14:1333–44.
Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDRþembryonic-stem-cell-derived population. Nature. 2008;453:524–8.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. https://doi.org/10.1038/nbt1393 .
Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21.
Burridge PW, Zambidis ET. Highly efficient directed differentiation of human induced pluripotent stem cells into cardiomyocytes. Methods Mol Biol. 2013;997:149–61.
Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39.
Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a selforganizing kidney. Nat Cell Biol. 2014;16:118–26.
Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, VunjakNovakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91.
Kadzik RS, Morrisey EE. Directing lung endoderm differentiation in pluripotent stem cells. Cell Stem Cell. 2012;10:355–61.
Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–97.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9.
Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–94.
Jun Cai, Ann DeLaForest, Joseph Fisher, Amanda Urick, Thomas Wagner, Kirk Twaroski, Max Cayo, Masato Nagaoka, Stephen A Duncan. Protocol for directed differentiation of human pluripotent stem cells toward a hepatocyte fate. 2012. DOI: https://doi.org/10.3824/stembook.1.52.1 .
Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, Rubin LL, Porter JA. Small-molecule modulators of hedgehog signaling: identification and characterization of smoothened agonists and antagonists. J Biol. 2002;1:10.
Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair celllike cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16.
Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–79.
Kshitiz PJ, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH. Control of stem cell fate and function by engineering physical microenvironments. Intergr Biol (Camb). 2012;4:1008–18.
Amps K, Andrews PW, Anyfantis G, Armstrong L, Avery S, Baharvand H, Baker J, Baker D, Munoz MB, Beil S, Benvenisty N, Ben-Yosef D, Biancotti JC, Bosman A, Brena RM, Brison D, Caisander G, Camarasa MV, Chen J, ChiaoE CYM, Choo AB, Collins D, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29:1132–44.
Nukaya D, Minami K, Hoshikawa R, Yokoi N, Seino S. Preferential gene expression and epigenetic memory of induced pluripotent stem cells derived from mouse pancreas. Genes Cells. 2015;20:367–81.
Murdoch A, Braude P, Courtney A, Brison D, Hunt C, Lawford-Davies J, Moore H, Stacey G, Sethe S, Procurement Working Group Of National Clinical H, E. S. C. F, National Clinical H, E. S. C. F. The procurement of cells for the derivation of human embryonic stem cell lines for therapeutic use: recommendations for good practice. Stem Cell Rev. 2012;8:91–9.
Hewitson H, Wood V, Kadeva N, Cornwell G, Codognotto S, Stephenson E, Ilic D. Generation of KCL035 research grade human embryonic stem cell line carrying a mutation in HBB gene. Stem Cell Res. 2016;16:210–2.
Daley GQ, Hyun I, Apperley JF, Barker RA, Benvenisty N, Bredenoord AL, Breuer CK, Caulfield T, Cedars MI, Frey-Vasconcells J, Heslop HE, Jin Y, Lee RT, Mccabe C, Munsie M, Murry CE, Piantadosi S, Rao M, Rooke HM, Sipp D, Studer L, Sugarman J, et al. Setting global standards for stem cell research and clinical translation: the 2016 ISSCR guidelines. Stem Cell Rep. 2016;6:787–97.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024 .
Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40.
Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, SuberbielleBoissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–7.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–16.
Ilic D, Ogilvie C. Concise review: human embryonic stem cells-what have we done? What are we doing? Where are we going? Stem Cells. 2017;35:17–25.
Rocha V, et al. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(1):34–4.
Longo UG, Ronga M, Maffulli N. Sports Med Arthrosc 17:112–126. Achilles tendinopathy. Sports Med Arthrosc. 2009;17:112–26.
Tempfer H, Lehner C, Grütz M, Gehwolf R, Traweger A. Biological augmentation for tendon repair: lessons to be learned from development, disease, and tendon stem cell research. In: Gimble J, Marolt D, Oreffo R, Redl H, Wolbank S, editors. Cell engineering and regeneration. Reference Series in Biomedical Engineering. Cham: Springer; 2017.
Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34.
Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–82.
Li R, Lin Q-X, Liang X-Z, Liu G-B, et al. Stem cell therapy for treating osteonecrosis of the femoral head: from clinical applications to related basic research. Stem Cell Res Therapy. 2018;9:291 https://doi.org/10.1186/s13287-018-1018-7 .
Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–9.
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–30.
Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplast. 2012;27(5):679–86.
Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, Lemaitre JM. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–53.
Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–8.
Petkovich DA, Podolskiy DI, Lobanov AV, Lee SG, Miller RA, Gladyshev VN. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 2017;25:954–60 https://doi.org/10.1016/j.cmet.2017.03.016 .
Gerontology, Rejuvenation by cell reprogramming: a new horizon in. Rodolfo G. Goya, Marianne Lehmann, Priscila Chiavellini, Martina Canatelli-Mallat, Claudia B. Hereñú and Oscar A. Brown. Stem Cell Res Therapy . 2018, 9:349. https://doi.org/10.1186/s13287-018-1075-y .
Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JLXJ, Rodriguez-Esteban C, Nuñez G, Nuñez Delicado E, Campistol JM, Guillen I, Guillen P, Izpisua. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167:1719–33.
de Lázaro I, Cossu G, Kostarelos K. Transient transcription factor (OSKM) expression is key towards clinical translation of in vivo cell reprogramming. EMBO Mol Med. 2017;9:733–6.
Sun S, Li ZQ, Glencer P, Cai BC, Zhang XM, Yang J, Li XR. Bringing the age-related macular degeneration high-risk allele age-related maculopathy susceptibility 2 into focus with stem cell technology. Stem Cell Res Ther. 2017;8:135 https://doi.org/10.1186/s13287-017-0584-4 .
Liu J. Induced pluripotent stem cell-derived neural stem cells: new hope for stroke? Stem Cell Res Ther. 2013;4:115 https://doi.org/10.1186/scrt326 .
Shahjalal HM, Dayem AA, Lim KM, Jeon TI, Cho SG. Generation of pancreatic β cells for treatment of diabetes: advances and challenges. Stem Cell ResTher. 2018;9:355 https://doi.org/10.1186/s13287-018-1099-3 .
Kroon E, Martinson LA, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26;443–52.
Arora V, Pooja A, Munshi AK. Banking stem cells from human exfoliated deciduous teeth. J Clin Pediatr Dent. 2009;33(4):289–94.
Mao JJ. Stem cells and the future of dental care. New York State Dental J. 2008;74(2):21–4.
Reznick, Jay B. Continuing education: stem cells: emerging medical and dental therapies for the dental Professional. Dentaltown Magazine . 2008, pp. 42–53.
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–95.
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith A. Dental pulp tissue engineering with stem cells from exfoliated. J Endod. 2008;34(8):962–9.
Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146(4):519–32. https://doi.org/10.1016/j.cell.2011.06.052 .
Sadri-Ardekani H, Atala A. Testicular tissue cryopreservation and spermatogonial stem cell transplantation to restore fertility: from bench to bedside. Stem Cell ResTher. 2014;5:68 https://doi.org/10.1186/scrt457 .
Zhang Q, Xu M, Yao X, Li T, Wang Q, Lai D. Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res Ther. 2015;6:152 https://doi.org/10.1186/s13287-015-0148-4 .
Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–82. https://doi.org/10.1038/cr.2009.56 .
Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell ResTher. 2010;1:37 https://doi.org/10.1186/scrt37 .
Wang Q, Matsumoto Y, Shindo T, Miyake K, Shindo A, Kawanishi M, Kawai N, Tamiya T, Nagao S. Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest. 2006;53:61–9. https://doi.org/10.2152/jmi.53.61 .
Article PubMed Google Scholar
Moghadam FH, Alaie H, Karbalaie K, Tanhaei S, Nasr Esfahani MH, Baharvand H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009;78:59–68. https://doi.org/10.1016/j.diff.2009.06.005 .
Byrne JA. Developing neural stem cell-based treatments for neurodegenerative diseases. Stem Cell ResTher. 2014;5:72. https://doi.org/10.1186/scrt461 .
Awe JP, Lee PC, Ramathal C, Vega-Crespo A, Durruthy-Durruthy J, Cooper A, Karumbayaram S, Lowry WE, Clark AT, Zack JA, Sebastiano V, Kohn DB, Pyle AD, Martin MG, Lipshutz GS, Phelps PE, Pera RA, Byrne JA. Generation and characterization of transgene-free human induced pluripotent stem cells and conversion to putative clinical-grade status. Stem Cell Res Ther. 2013;4:87. https://doi.org/10.1186/scrt246 .
Peng J, Zeng X. The role of induced pluripotent stem cells in regenerative medicine: neurodegenerative diseases. Stem Cell ResTher. 2011;2:32. https://doi.org/10.1186/scrt73 .
Wright BL, Barker RA. Established and emerging therapies for Huntington’s disease. 2007;7(6):579–87 https://www.ncbi.nlm.nih.gov/pubmed/17896994/579-87 .
Lin NH, Gronthos S, Bartold PM. Stem cells and periodontal regeneration. Aust Dent J. 2008;53:108–21.
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55.
Ramseier CA, Abramson ZR, Jin Q, Giannobile WV. Gene therapeutics for periodontal regenerative medicine. Dent Clin North Am. 2006;50:245–63.
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. OrthodCraniofac Res. 2005;8:191–9.
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein. J Dent Res. 2004;83:590–5.
Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haikel Y, et al. Bone marrow cells can give rise to ameloblast-like cells. J Dent Res. 2006;85:416–21.
Liu Y, Liu W, Hu C, Xue Z, Wang G, Ding B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells. 2011;29(11):1804–16. https://doi.org/10.1002/stem.728 .
Raspini G, Wolff J, Helminen M, Raspini G, Raspini M, Sándor GK. Dental stem cells harvested from third molars combined with bioactive glass can induce signs of bone formation in vitro. J Oral Maxillofac Res. 2018;9(1):e2. Published 2018 Mar 31. https://doi.org/10.5037/jomr.2018.9102 .
Christodoulou I, Goulielmaki M, Devetzi M, Panagiotidis M, Koliakos G, Zoumpourlis V. Mesenchymal stem cells in preclinical cancer cytotherapy: a systematic review. Stem Cell Res Ther. 2018;9(1;336). https://doi.org/10.1186/s13287-018-1078-8 .
Bansal R, Jain A. Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med. 2015;6(1):29–34. https://doi.org/10.4103/0976-9668.149074 .
Article PubMed PubMed Central Google Scholar
Edgar Ledesma-Martínez, Víctor Manuel Mendoza-Núñez, Edelmiro Santiago-Osorio. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells Int . 2016, 4,709,572, p. doi: https://doi.org/10.1155/2016/4709572 ].
Grzech-Leśniak K. Making use of lasers in periodontal treatment: a new gold standard? Photomed Laser Surg. 2017;35(10):513–4.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12. https://doi.org/10.1073/pnas.0937635100 .
Yasui T, Mabuchi Y, Toriumi H, Ebine T, Niibe K, Houlihan DD, Morikawa S, Onizawa K, Kawana H, Akazawa C, Suzuki N, Nakagawa T, Okano H, Matsuzaki Y. Purified human dental pulp stem cells promote osteogenic regeneration. J Dent Res. 2016;95(2):206–14. https://doi.org/10.1177/0022034515610748 .
Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res. 2014;78:16–20. https://doi.org/10.1016/j.neures.2013.10.010 .
d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;12, PMID: 19908196:75–83.
Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, Yang Q, Wang C, Duan Y, Jin Y. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng. Part A. 2011;17(7–8):1015–26. https://doi.org/10.1089/ten.tea.2010.0140 .
Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, Feijen J, Okano T, Ishikawa I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37(12):1088–99. https://doi.org/10.1111/j.1600-051X.2010.01597.x .
Chen F-M, Gao L-N, Tian B-M, Zhang X-Y, Zhang Y-J, Dong G-Y, Lu H, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. https://doi.org/10.1186/s13287-016-0288-1 .
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56(7):709–21. https://doi.org/10.1016/j.archoralbio.2010.12.008 .
Han C, Yang Z, Zhou W, Jin F, Song Y, Wang Y, Huo N, Chen L, Qian H, Hou R, Duan Y, Jin Y. Periapical follicle stem cell: a promising candidate for cementum/periodontal ligament regeneration and bio-root engineering. Stem Cells Dev. 2010;19(9):1405–15. https://doi.org/10.1089/scd.2009.0277 .
Luan X, Ito Y, Dangaria S, Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15(4):595–608. https://doi.org/10.1089/scd.2006.15.595 .
Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, Sato S, Toyoda M, Teranaka T, Narayanan AS. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res. 2002;43(2–3):406–8 PMID: 12489190.
Guo W, Chen L, Gong K, Ding B, Duan Y, Jin Y. Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Engineering. Part A. 2012;18(5–6):459–70 https://doi.org/10.1089/ten.tea.2011.0261 .
Bai, Yudi et al. Cementum- and periodontal ligament-like tissue formation by dental follicle cell sheets co-cultured with Hertwig’s epithelial root sheath cells. Bone. 2011, 48, Issue 6, pp. 1417–1426, https://doi.org/10.1016/j.bone.2011.02.016 .
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. 2008, 34, pp. 962–969.
Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate, hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res 2. 2002;43:387–90.
Friedlander LT, Cullinan MP, Love RM. Dental stem cells and their potential role in apexogenesis and apexification. Int Endod J. 2009;42:955–62.
Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, Li A, Huang K, Luo R, Wang L, Liu Y, Zhou T, Wei S, Pan G, Pei D, Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen (Lond). July 30, 2013, 2(1), pp. 6, doi: https://doi.org/10.1186/2045-9769-2-6 .
Craig J. Taylor, Eleanor M. Bolton, and J. Andrew Bradley 2011 Aug 12 and https://doi.org/10.1098/rstb.2011.0030 ], 366(1575): 2312–2322. [doi:. Immunological considerations for embryonic and induced pluripotent stem cell banking,. Philos Trans R SocLond B Biol Sci. 2011, 366(1575), pp. 2312–2322, doi: https://doi.org/10.1098/rstb.2011.0030 .
T.R. Nayak, H. Andersen, V.S. Makam, C. Khaw, S. Bae, X.F. Xu, P.L.R. Ee, J.H. Ahn, B.H. Hong, G. Pastorin, B. Ozyilmaz, ACS Nano, 5 (6) (2011), pp. 4. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells,. ACS Nano. 2011, pp. 4670–4678.
Lee WC, Lim C, Shi H, Tang LAL, Wang Y, Lim CT, Loh KP. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–41.
Kenry LWC, Loh KP, Lim CT. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236–50.
Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. 2009. 2009, 4(3), p. https://doi.org/10.1371/journal.pone . 0004722.
Oh, Myeongsik, et al. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19(6), p. 1715.
Ramirez MI. et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881–906.
Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–9.
Mateescu B, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—an ISEV position paper. J. Extracell. Vesicles. 2017;6(1). https://doi.org/10.1080/20013078.2017.1286095 .
Nawaz M, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:17. Article ID 1073140.
Helfrich, Y.R., Sachs, D.L. and Voorhees, J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 20, pp. 177–183, https://www.ncbi.nlm.nih.gov/pubmed/18649702 .
Julia Tigges, Jean Krutmann, Ellen Fritsche, Judith Haendeler, Heiner Schaal, Jens W. Fischer, Faiza Kalfalah, Hans Reinke, Guido Reifenberger, Kai Stühler, Natascia Ventura, Sabrina Gundermann, Petra Boukamp, Fritz Boege. The hallmarks of fibroblast ageing, mechanisms of ageing and development, 138, 2014, Pages 26–44. 2014, 138, pp. 26–44, ISSN 0047–6374, https://doi.org/10.1016/j.mad.2014.03.004 .
Huh MI, Kim MS, Kim HK, et al. Effect of conditioned media collected from human amniotic fluid-derived stem cells (hAFSCs) on skin regeneration and photo-aging. Tissue Eng Regen Med. 2014;11:171 https://doi.org/10.1007/s13770-014-0412-1 .
Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31.
Liu J, Han G, Liu H, et al. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PLoS One. 2013;8:e62844.
Oh M, et al. Promotive effects of human induced pluripotent stem cell-conditioned medium on the proliferation and migration of dermal fibroblasts. Biotechnol. Bioprocess Eng. 2017;22:561–8.
Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One. 2008;3:e1886.
Bae J-S, Lee S-H, Kim J-E, Choi J-Y, Park R-W, Park JY, Park H-S, Sohn Y-S, Lee D-S, Lee EB. βig-h3 supports keratinocyte adhesion, migration, and proliferation through α3β1 integrin. Biochem. Biophys. Res. Commun. 2002;294:940–8.
Zhou B-R, Xu Y, Guo S-L, Xu Y, Wang Y, Zhu F, Permatasari F, Wu D, Yin Z-Q, Luo D. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. BioMed Res. Int. 2013;519:126.
Peng Y, Baulier E, Ke Y, Young A, Ahmedli NB, Schwartz SD, et al. Human embryonic stem cells extracellular vesicles and their effects on immortalized human retinal Müller cells. PLoS ONE. 2018, 13(3), p. https://doi.org/10.1371/journal.pone.019400 .
Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003.
Mascetti VL, Pedersen RA. Human-mouse chimerism validates human stem cell pluripotency. Cell Stem Cell. 2016;18:67–72.
Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepúlveda P. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2007;26(3):638–45.
Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14(2):149–56.
Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–23.
de Mendonça CA, Bueno DF, Martins MT, Kerkis I, Kerkis A, Fanganiello RD, Cerruti H, Alonso N, Passos-Bueno MR. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg. 2008;19(1):204–10.
Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, Lee JS, Shi S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14(5):428–34.
Abbas, Diakonov I., Sharpe P. Neural crest origin of dental stem cells. Pan European Federation of the International Association for Dental Research (PEF IADR). 2008, Vols. Seq #96 - Oral Stem Cells.
Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Gaiad TP, Morini AC, Vieira NM, Marina P, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs. J Transl Med. 2008;6:35.
Xianrui Yang, Li Li, Li Xiao, Donghui Zhang. Recycle the dental fairy’s package: overview of dental pulp stem cells. Stem Cell Res Ther . 2018, 9, 1, 1. https://doi.org/10.1186/s13287-018-1094-8 .
Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010;19:1375–83.
Wang J, et al. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly (L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010;6(10):3856–63.
Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J Bone Miner Metab. 2015;33:371–82.
Huang GT-J, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–73.
Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29(6):532–9.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30.
Nuti N, Corallo C, Chan BMF, Ferrari M, Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep. 2016;12:511–23.
Ferro F, et al. Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PloS One. 2012;7(7):e41774.
Conde MCM, Chisini LA, Grazioli G, Francia A, Carvalho RVd, Alcázar JCB, Tarquinio SBC, Demarco FF. Does cryopreservation affect the biological properties of stem cells from dental tissues? A systematic review. Braz Dent J. 2016;1210(6):633-40. https://doi.org/10.1590/0103-6440201600980 .
Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319–25.
Alge DL, Zhou D, Adams LL, et. al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4(1):73–81.
Jo Y-Y, Lee H-J, Kook S-Y, Choung H-W, Park J-Y, Chung J-H, Choung Y-H, Kim E-S, Yang H-C, Choung P-H. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5.
Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005;20:1394–402.
Zainal A, Shahrul H, et al. In vitro chondrogenesis transformation study of mouse dental pulp stem cells. Sci World J. 2012;2012:827149.
Wei X, et al. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33(6):703–8.
Dai J, et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials. 2012;33(31):7699–711.
Vasandan AB, et al. Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. J Cell Mol Med. 2014;18(2):344–54.
Werle SB, et al. Carious deciduous teeth are a potential source for dental pulp stem cells. Clin Oral Investig. 2015;20:75–81.
Nemeth CL, et al. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng A. 2014;20(21–22):2817–29.
Paino F, Ricci G, De Rosa A, D’Aquino R, Laino L, Pirozzi G, et al. Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. Eur. Cell Mater. 2010;20:295–305.
Ferro F, Spelat R, Baheney CS. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. In: Kioussi C, editor. Stem cells and tissue repair. Methods in molecular biology (methods and protocols): Humana Press. 2014;1210.
Ishkitiev N, Yaegaki K, Imai T, Tanaka T, Nakahara T, Ishikawa H, Mitev V, Haapasalo M. High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J Endod. 2012;38:475–80.
Download references
Acknowledgements
Not applicable.
This work is supported by Wrocław Medical University in Poland.
Availability of data and materials
Please contact author for data requests.
Author information
Authors and affiliations.
Department of Experimental Surgery and Biomaterials Research, Wroclaw Medical University, Bujwida 44, Wrocław, 50-345, Poland
Wojciech Zakrzewski, Maria Szymonowicz & Zbigniew Rybak
Department of Conservative Dentistry and Pedodontics, Krakowska 26, Wrocław, 50-425, Poland
Maciej Dobrzyński
You can also search for this author in PubMed Google Scholar
Contributions
WZ is the principal author and was responsible for the first draft of the manuscript. WZ and ZR were responsible for the concept of the review. MS, MD, and ZR were responsible for revising the article and for data acquisition. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Wojciech Zakrzewski .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M. et al. Stem cells: past, present, and future. Stem Cell Res Ther 10 , 68 (2019). https://doi.org/10.1186/s13287-019-1165-5
Download citation
Published : 26 February 2019
DOI : https://doi.org/10.1186/s13287-019-1165-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Differentiation
- Pluripotency
- Induced pluripotent stem cell (iPSC)
- Stem cell derivation
- Growth media
- Tissue banks
- Tissue transplantation
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Revolutionizing medicine: recent developments and future prospects in stem-cell therapy
Bashdar m hussen , phd, mohammad taheri , phd, raya kh yashooa , msc, gaylany h abdullah , phd, snur r abdullah , bsc, ramiar kamal kheder , msc, suhad a mustafa , phd.
- Author information
- Article notes
- Copyright and License information
Corresponding author. Address: General Directorate of Scientific Research Center, Salahaddin University-Erbil, Kurdistan Region, Erbil, Iraq. Tel.: +964 750 783 55 66. E-mail: [email protected] (S.A. Mustafa).
Received 2024 Apr 4; Accepted 2024 Sep 27; Collection date 2024 Dec.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal. http://creativecommons.org/licenses/by-nc-nd/4.0/
Stem-cell therapy is a revolutionary frontier in modern medicine, offering enormous capacity to transform the treatment landscape of numerous debilitating illnesses and injuries. This review examines the revolutionary frontier of treatments utilizing stem cells, highlighting the distinctive abilities of stem cells to undergo regeneration and specialized cell differentiation into a wide variety of phenotypes. This paper aims to guide researchers, physicians, and stakeholders through the intricate terrain of stem-cell therapy, examining the processes, applications, and challenges inherent in utilizing stem cells across diverse medical disciplines. The historical journey from foundational contributions in the late 19th and early 20th centuries to recent breakthroughs, including ESC isolation and iPSC discovery, has set the stage for monumental leaps in medical science. Stem cells’ regenerative potential spans embryonic, adult, induced pluripotent, and perinatal stages, offering unprecedented therapeutic opportunities in cancer, neurodegenerative disorders, cardiovascular ailments, spinal cord injuries, diabetes, and tissue damage. However, difficulties, such as immunological rejection, tumorigenesis, and precise manipulation of stem-cell behavior, necessitate comprehensive exploration and innovative solutions. This manuscript summarizes recent biotechnological advancements, critical trial evaluations, and emerging technologies, providing a nuanced understanding of the triumphs, difficulties, and future trajectories in stem cell-based regenerative medicine. Future directions, including precision medicine integration, immune modulation strategies, advancements in gene-editing technologies, and bioengineering synergy, offer a roadmap in stem cell treatment. The focus on stem-cell therapy’s potential highlights its significant influence on contemporary medicine and points to a future in which individualized regenerative therapies will alleviate various medical disorders.
Keywords: biotechnology advancements, clinical trials, medical revolution, stem-cell therapy
Stem cell therapy represents a groundbreaking frontier in modern medicine, offering unprecedented potential to address a wide range of debilitating diseases and injuries.
Stem cells possess unique properties, including self-renewal and differentiation into specialized cell types, making them indispensable for regenerative medicine applications.
The historical journey of stem cell research, from foundational contributions in the late 19th and early 20th centuries to recent breakthroughs like the isolation of embryonic stem cells and induced pluripotent stem cells, highlights the monumental progress in medical science.
Stem cell therapy holds promise for treating various conditions, including cancer, neurodegenerative disorders, cardiovascular diseases, spinal cord injuries, diabetes, and tissue damage.
Despite the immense potential, stem cell therapy faces challenges such as immune rejection, tumorigenesis, and the precise manipulation of stem cell behaviors, necessitating innovative solutions for clinical translation.
Recent biotechnological advancements, such as exosome-based therapeutics, single-cell RNA sequencing, and CRISPR technology, have revolutionized stem cell research, offering new opportunities for precise genome editing and therapeutic interventions.
Regulatory considerations are paramount in the clinical translation of stem cell therapies, requiring adherence to strict guidelines and directives to ensure safety and efficacy.
The future of stem cell therapy lies in precision medicine integration, immune modulation strategies, advancements in gene editing technologies, and synergies with bioengineering, paving the way for continued evolution and personalized regenerative therapies.
Stem-cell therapy signifies a pioneering frontier in modern medicine that uses the extraordinary power of stem cells and their revolutionary potential to treat diverse illnesses. Stem cells play a crucial role in regenerative medicine and exhibit the extraordinary ability to differentiate into various cell types and to renew themselves. Their intrinsic capacity to repair and regenerate tissues holds immense promise for revolutionizing therapeutic interventions 1 , 2 . The historical journey of stem-cell investigation can be traced to pivotal contributions from visionaries such as Boveri, Häcker, Maximow, and Cohnheim during the late 19th and early 20th centuries 3 . Their foundational work placed the groundwork for comprehension of the fundamental principles of stem cells and for shedding light on their roles in developmental processes and tissue repair. These early insights have laid the foundation for contemporary stem-cell investigations, fueling a deeper exploration of their biological significance 3 , 4 . Important turning points in the history of this field include the identification of ESCs in 1981 by Kaufman and Evans 5 – 7 and Thomson’s discovery of iPSCs in 2007 8 . Although stem-cell therapies have vast and promising potential, several challenges and complexities loom in their clinical translation 9 . Issues like immunological rejection, tumorigenesis, and precise manipulation of stem-cell behavior for optimal therapeutic outcomes are critical hurdles that necessitate comprehensive exploration and innovative solutions 1 , 10 – 12 . Advances in biotechnology, especially the revolution in exosome-based therapeutics, single-cell RNA sequencing (scRNA-Seq), and CRISPR technology 13 – 15 , one of the major developments in genetic engineering, has made precise and effective genome editing possible, which opens new avenues for modified genetic material, leading to advances in a variety of fields such as biotechnology and medicine 16 , 17 . Regenerative medicine represents a novel and promising therapeutic approach for individuals with exhausted or nonexistent options for managing their medical condition. Research studies, such as identification, clinical trials, and therapeutic applications on stem-cell have been extensive in recent years because of promising results from preclinical research (Fig. 1 ). The process of bringing these novel medicinal items from laboratories to the market is governed by strict guidelines and directives issued by qualified regulatory bodies 18 . Stem cells can be obtained for tissue engineering and cell treatments from four primary sources. The stem cells primary sources are embryonic and fetal tissues, comprising the placenta (including the chorion and amnion), umbilical cord (Wharton jelly), and particular tissues inside the adult, such as blood, skin, skeletal muscle, fat, and bone marrow, and somatic cells that have undergone genetic reprogramming to become distinct from their original state, such as iPSCs 19 .
A timeline depicting the introduction of mesenchymal stem cells (MSCs), their early research, and their substantial application in clinical trials, immunoregulation, and disease treatment.
Through an extensive synthesis of recent biotechnological advancements, critical evaluations, and emerging technologies, this review offers a nuanced comprehension of the advantages, difficulties, and future trajectories of stem cell-based regenerative therapy. By examining the historical foundations, current landscape, and prospects, this study endeavors to serve as a guide for researchers, clinicians, and stakeholders in navigating the intricate terrain of stem-cell therapy.
Search strategy
An extensive examination of existing literature was performed using the Embase, Web of Science, PubMed, and Scopus databases. The terms ‘stem cell therapy’, ‘medical revolution’, ‘biotechnology advancements’, and ‘clinical trial’ were used in the search. Only articles published in English were included in the search. We assessed the abstracts of each article to determine the relevance of the retrieved papers to the topic. Subsequently, every relevant paper ( in vivo , in vitro , and human-based research) was selected as part of the study.
Stem-cell types
Embryonic stem cells (escs).
ESCs exhibit characteristics that distinguish them from each other in stem cell biology. Notably, their pluripotency, which is defined by distinct features to differentiate into any human body cell, makes them highly adaptable and has great therapeutic promise 20 . Additionally, ESCs have a notably high self-renewal capacity, which contributes to their sustained presence and functionality over extended periods 21 . Potential ESC sources include mice, nonhuman primates, and humans. They are isolated from the blastocysts’ inner cell mass before implantation 22 , 23 . Because they are pluripotent cells, they can produce various kinds of cells from fetuses and adults in vivo and in vitro 24 – 26 . Two methods were employed to separate ESCs from blastocysts’ inner cell masses. Microsurgery is the most commonly used surgical approach. Mechanical dissection in the microscopic direction is used to isolate cells of the trophoblastic lineage from the rest of the cell mass. The second approach entails employing an antibody to target trophoblast lineage cells 27 , 28 .
Regarding potential applications, the pluripotent nature of ESCs opens avenues for significant contributions to tissue regeneration and repair. Their capacity to undergo differentiation into many cell lineages holds promise for treating degenerative conditions and injuries, making them pivotal players in regenerative medicine. Furthermore, ESCs serve as invaluable tools in disease modeling for research purposes 29 . By replicating specific cellular environments, researchers can discover more about the workings of various disorders, providing a framework for cellular disease research and aiding in the creation of focused therapies. The unique properties of ESCs are relevant to drug testing and development 30 . Because of their pluripotency, a variety of cell populations can be created to provide a more complete picture of human cellular responses. This capability is particularly valuable for evaluating drug efficacy and safety and provides a sophisticated model for preclinical testing. Consequently, the multifaceted potential of ESCs dramatically enhances our comprehension of biology, fostering medical research and shaping the landscape of therapeutic innovation 31 , 32 .
Adult stem cells (ASCs)
ASCs stand out in the realm of regenerative biology because of their distinctive properties and vital roles in maintaining tissue homeostasis 33 . Multipotency is the ability of cells to possess various potential fates or abilities to develop into a restricted, diverse array of cellular phenotypes 34 . ASCs are endogenous stem cells that are crucial for preserving the tissues’ structural integrity, like bone, skin, and blood. They are located in specific niches or tissue sections 35 . ASCs have been discovered in several tissues, including blood, stomach, muscle, skin, brain, and heart 36 . They are less potent than ESCs; however, they have demonstrated efficacy in disease treatment. They can be extracted and harvested from individuals and used for tissue regeneration through autologous or allogeneic transplantation 37 . ASCs have a more specialized differentiation capability than pluripotent cells, such as ESCs, and can help generate particular cell lineages within their original tissue 34 .
Stem cells’ function in repairing damaged tissues and maintenance is essential throughout an individual’s lifespan 38 . The unique ability of ASCs to maintain tissue and exhibit multipotency lends itself to a variety of possible uses within the regenerative medicine field 39 . Tissue-specific regeneration and repair are among the most promising approaches. ASCs can be utilized to regenerate damaged or deteriorated tissues due to their presence in diverse tissues, including the bone marrow, skin, and muscle 40 . Their capacity to undergo cell type-specific differentiation that is relevant to their native tissues places them at the forefront of tailored regeneration techniques, offering potential treatment options for ailments ranging from degenerative illnesses unique to certain organs to musculoskeletal injuries 41 .
ASCs are an appealing therapeutic choice for degenerative diseases. Because of their functions in tissue repair and regeneration, they are desirable targets for therapies aimed at slowing the advancement of illnesses marked by cellular degeneration 42 . Through the utilization of the regenerative capacity of these cells, scientists and medical professionals have investigated ways to create novel treatments that target the root causes of degenerative illnesses with the aim of enhancing patient outcomes and quality of life 43 . Within the class of ASCs, hematopoietic stem cells are a specific subset essential for bone marrow transplantation 44 . The immune system and blood regeneration rely on hematopoietic stem cells (HSCs), which are essential due to their versatility in cell differentiation into various blood cell types 45 . The utilization of these cells in bone marrow transplants represents a cornerstone in hematological therapies, offering a curative approach for conditions like leukemia and other disorders affecting the blood and immune systems 46 , 47 . Transplantation of hematopoietic stem cells is a life-saving intervention that reinstates functional blood and immune cell populations in individuals with hematopoietic disorders 48 .
Perinatal stem cells
Embryonic stem cells are derived from the amniotic fluid, placenta, and umbilical cord and represent a unique category within the spectrum of stem cell types 49 . Fetal cells possess multipotent capabilities and can differentiate into a restricted type of cells 50 . These cells are distinctively derived from tissues associated with the prenatal and perinatal stages of development, indicating their specialized origin 49 . Notably, perinatal stem cells exhibit a hybrid nature, sharing characteristics analogous to those of adults and ESCs. Their dual features make them adaptable and potentially useful for various regenerative medicine applications 51 . Perinatal stem cells offer a noncontroversial and ethically sound reservoir for therapeutic purposes 49 . Their properties, which are reminiscent of those of ESCs and ASCs, contribute to their unique regenerative potential. Since these cells undergo cell differentiation into a wide variety of cells, tailored approaches for tissue regeneration and repair are possible 52 . Perinatal stem cells show promise in furthering regenerative medicine across a range of tissues in terms of prospective uses. They are important components in targeted tissue renewal because of their capacity to specialize in particular cell lineages 52 .
Moreover, its therapeutic potential can be extended to other conditions, such as cerebral palsy and diabetes. Perinatal stem cells offer a novel and innovative approach to the development of medicines tailored to address the complexities of these disorders by exploiting their regenerative properties and versatile differentiation capabilities 53 . One notable advantage of perinatal stem cells is their potential for allogeneic transplantation without eliciting immune rejection. The immunomodulatory characteristics of these cells make them well-suited for transplantation across different individuals, eliminating the need for a perfect match between the donor and recipient 54 . This opens new possibilities for allogeneic stem-cell therapies, providing a feasible and practical approach to transplantation procedures without the intricate challenges associated with immune compatibility.
In summary, perinatal stem cells signify a distinct and highly promising category of stem cells with hybrid properties. Their application in regenerative medicine, therapeutic interventions for specific conditions, and allogeneic transplantation underscore their potential to reshape the landscape of stem cell-based therapies.
Induced pluripotent stem cells (iPSCs)
The iPSCs represent a revolutionary category in stem-cell studies and are characterized by properties that mirror those of ESCs 55 . Several human and mouse investigations have utilized fibroblasts and skin cells as the primary sources of adult cells. It has been discovered that adult brain stem cells have been identified as the primary cell type in investigations of reprograming cells 56 . Another study reported that murine bone marrow mononuclear cells can be reprogrammed more effectively than mouse embryonic fibroblasts 57 . Notably, iPSCs and their embryonic counterparts possess the capacity to undergo pluripotency to differentiate into distinct kinds of specialized cells 58 . One important way to iPSCs is to distinguish them from ESCs by their source, in which in order to create iPSCs, adult cells are reprogrammed. This methodology provides a novel means of addressing ethical concerns regarding the use of ESCs in scientific investigation 59 . Personalized medicine could undergo significant transformations if adult cells are reprogrammed to become iPSCs. The advancement of individualized cellular therapeutics involves the process of cellular reprogramming for individual patients is one of the main uses of iPSCs 60 . The iPSCs have the remarkable ability to transform into a wide variety of disease-specific cell types during cell reprogramming. This personalized approach improves the integrity and efficiency of cell-based treatments and offers a potential path in order to treat numerous illnesses and traumas.
Furthermore, iPSCs play a pivotal role in disease modeling in personalized medicine 61 . The capacity to generate iPSCs from individuals with particular genetic conditions has enabled researchers to create in vitro disease models. These models are extremely invaluable tools for understanding disease mechanisms at the cellular level and enable the exploration of targeted therapeutic interventions 62 . iPSC-based disease modeling advances the field of personalized medicine by enabling a more accurate and customized approach to medical research, thus opening the door for customized treatments. Beyond illness modeling and customized treatments, iPSCs have a major impact on toxicity assessments and drug development 63 . The pluripotent characteristics of iPSCs allow the generation of diverse cellular phenotypes, providing a flexible platform for evaluating the safety and effectiveness of pharmaceuticals. iPSC-based assays offer a more thorough understanding of how pharmaceuticals interact with various cell types, which helps identify possible side effects and directs advancements in the creation of remedies that are both safer and more effective 64 .
In conclusion, iPSCs offer a revolutionary approach to stem-cell investigation, owing to their pluripotent characteristics and the origin of adult cell reprogramming. Their applications in patient-specific cell therapies, disease modeling for personalized medicine, and drug discovery underscore their potential to revolutionize medical treatment and contribute to advancements in personalized healthcare.
Stem cells mechanisms of action
Stem cells secrete numerous factors and exosomes that are responsible for immunomodulatory, antiapoptotic, antibacterial, and microbial properties. In addition to the ability for repair, communication, and regeneration (Fig. 2 ).
The schematic diagram represents the mesenchymal stem cells’ mechanism of action and their interaction with immune cells, including differentiation, immunomodulation, antiapoptotic effects, exosome and microvesicle release, migration and homing, and matrix remodeling.
Stem cells’ immunomodulatory actions have undergone extensive research when contrasted with other stem cell types 65 , 66 . Stem cells have a role in suppressing acute-phase responses by suppressing excessive activation of macrophages and T cells and initiating the secretion of inflammatory cytokines. This could decrease the likelihood of a cytokine storm 67 . Toll-like receptors (TLRs) present in MSCs detect injury signals and initiate immunomodulatory responses 68 . MSCs exhibit immunomodulatory properties via paracrine activity and direct intercellular communication facilitated by several bioactive compounds like cytokines, chemokines, and growth factors. These molecules affect both adaptive and innate immunity. MSCs can prevent the activation of T-cells via several immunomodulatory substances, such as TGF-β1, PGE2, and HLA-G5. They also utilize molecules that are linked to a membrane, such as VCAM-1, PD-L1, and Gal-1 69 , 70 . MSCs regulate NK cell cytotoxicity by reducing the expression of IFN-γ 71 . Cytokines are crucial for preserving the ability of ESCs to reproduce. This is achieved through the action of a specific cytokine called leukemia inhibitory factor (LIF), which belongs to the class of cytokines known as interleukin-6 72 . The iPSCs can modulate the immune system, as demonstrated through their capacity to suppress the rapid increase of responder T cells in modified combined leukocyte reactions in vitro 73 .
In addition, apoptosis serves as a protective process within the immunological response of the host to combat pathogens and has a crucial function in interactions between the host and pathogens 71 . MSCs can inhibit apoptosis, which may occur due to pathogens, low oxygen levels, mechanical stress, or radiation. For instance, the ability of MSCs to avoid cell death (antiapoptotic effects) has been investigated in cardiac ischemia, neurological conditions, and respiratory ailments 74 . In addition, during apoptosis caused by hypoxia, MSCs stimulate the expression of certain proteins, including HGF, VEGF, and TGF-β1, with the potential to prevent endothelial cell death 75 . Additional variables contribute to the antiapoptotic effect of MSCs, such as IL-6 and IGF-1, which results in enhanced secretion of SFRP2 protein 76 .
Stem cells exert their antimicrobial activity by secreting molecules and direct cell-to-cell interactions, namely by releasing antimicrobial peptides (AMPs). The antimicrobial activities are carried out by specific AMPs like the family of lipocalins (Lcn2), hepcidin, and b-defensins (hBD-1, hBD-2, and hBD-3) 77 , 78 . Stem cells boost their antimicrobial activity by upregulating LL-37, a peptide that is stimulated by bacteria and inhibits bacterial growth 79 .
Regeneration and restoration of damaged tissues rely heavily on stem cells because of their distinctive ability to suppress aberrant immune responses, their capacity to transform into specific tissues, and produce certain substances that stimulate the host’s reparative and regenerative systems 80 . Furthermore, the micro-vesicles and exosomes generated from stem cells are important for stem-cell communication and regeneration. Lipids, proteins, nucleic acids, including RNA and micro RNA, and signaling molecules are among the many bioactive compounds that are transported within the extracellular vesicles (EVs) emitted by stem cells of the body 81 . Compounds secreted by stem cells facilitate tissue regeneration by promoting the growth and specialization of stem/progenitor cells in the immediate vicinity. In addition, they control the placement of molecules in the extracellular matrix, activate pathways that prevent scarring, and promote the development of new blood vessels 82 , 83 . MSCs release soluble paracrine factors, including ANGPT1, HGF, EGF, VEGF, KGF, PGE2, and interleukin-10 (IL10). These factors can improve the restoration of epithelial and endothelial cells 84 , 85 .
Recent advancements in stem-cell research
Recent years have seen remarkable progress in stem-cell research that has greatly expanded our comprehension of stem-cell biology 86 . One notable milestone was the elucidation of novel mechanisms governing stem cell fate decisions. Researchers have uncovered key signaling pathways and transcription factors that play pivotal roles in directing stem-cell differentiation 87 , 88 . A cellular communication system known as the Notch signaling pathway is vital for various physiological and developmental functions 89 . Researchers have demonstrated the significance of the Notch pathway in determining the outcome of cells by either promoting the renewal of cells or their differentiation into various types of stem cells, including ESCs 90 , PSCs 91 , HSCs 92 , NSCs 93 , and ISCs 94 . Other instances of the signaling pathways are the PI3k/AKT signaling 95 and TGF-β signaling 96 . A transcription factor known as NF-κB controls the diverse functions of NF-κB in stem cells and developmental processes 97 . These findings enhance stem cell manipulation capabilities for specific therapeutic purposes, offering unprecedented opportunities for targeted cell-based interventions 98 . Recent studies have explored the nuances of lineage commitment and cellular specialization within the framework of stem-cell development. Scientists have identified regulatory networks that govern stem cell differentiation into distinct cell types, shedding light on the molecular events that dictate cell fate 99 , 100 .
Researchers have also unveiled insights into the epigenetic modifications associated with reprogramming, enhancing our comprehension of the molecular mechanisms by which somatic cells transform into pluripotent states 101 . For example, studies proved that gene expression and cellular identity are influenced by changes in DNA methylation patterns during the formation of iPSCs 102 . Modification of histones through acetylation and methylation, which affect chromatin structure and gene regulation, also play significant roles in reprogramming. This new understanding of epigenetic pathways helps clarify the complex processes involved in pluripotency induction and cellular reprogramming.
These advancements have contributed to improvements in iPSC-based methods for pharmaceutical innovation, disease modeling, and customized regenerative medicine 62 . Another significant stride in stem-cell research pertains to the tissue regeneration field 103 .
Transplantation of stem cells has great potential as a medicine applied to numerous illnesses. In neurology clinical trials, scientists are presently investigating stem cell therapy’s feasibility for the purpose of alleviating neurological disorders, such as Alzheimer’s and Parkinson’s 104 . Additionally, investigations are being conducted on stem-cell therapy for cardiovascular illnesses, orthopedic conditions, hematological conditions, and diabetes. The adaptability of stem cells, coupled with advancements in delivery techniques, positions them as potential game-changers in regenerative medicine 105 . Emerging applications include the use of stem cells in immunotherapy, where they are engineered to target and treat certain cancers 106 . Furthermore, continuous investigations have investigated the possibility of using stem cells to regulate the immune system in disorders like autoimmune illnesses 107 . As these clinical applications progress from research to practice, the landscape of healthcare is poised to undergo significant transformation.
Neural stem-cell transplants have been administered to patients with PD in a clinical trial. In addition to improving motor system function, the data demonstrated a slowing of the disease’s progression and suggested the prospects of stem cells for neurological regeneration 108 , 109 . Individuals with heart failure participated in a cardiac stem-cell clinical trial. The outcomes showed less scar tissue, increased angiogenesis, and improved heart function, indicating the effectiveness of stem-cell treatment in promoting the regrowth of cardiac tissue 110 , 111 . Additionally, bone marrow-derived MSCs (BM-MSCs) have been utilized in a clinical study of osteoarthritis. Patients experience decreased pain, improved joint function, and evidence of cartilage regeneration, demonstrating the therapeutic prospects of stem cells in orthopedic applications 112 – 114 .
Treating leukemia with HSC transplantation (HSCT) has proven beneficial. Patients undergoing this procedure achieve complete remission and hematopoietic system reconstitution, leading to prolonged survival and improved quality of life 46 , 115 . Furthermore, clinical trials utilizing iPSCs to generate pancreatic progenitor cells have demonstrated promise for the treatment of diabetes. Patients exhibit restored insulin production and improved glycemic control, suggesting a regenerative approach to diabetes management 116 , 117 (Table 1 ) (Fig. 3 ).
The advancement in stem-cell therapies in various diseases.
MSC sources, such as bone marrow, adipose tissue, and placenta, and their role in the therapy of different diseases. MSCs improve and combat diseases including pneumonia, leukemia, neuron diseases, osteoarthritis hear diseases, and the two types of diabetes. MSCs have immunoregulator and anti-inflammatory properties.
In combating the COVID-19 pandemic, universal vaccination remains the primary strategy; however, uncertainties persist regarding the duration of vaccine protection and the inability of any vaccine to provide absolute immunity 137 . Stem-cell therapy has arisen as a potential substitute, building on successes observed in severe H7N9 avian influenza 138 , 139 . Stem cells, particularly those derived from human umbilical cord stem cells (hUCMSCs), are effective and safe for treating severe COVID-19, demonstrating their potential in over 100 international clinical trials 140 . Allogeneic MSCs, notably hUCMSCs, contribute to anti-inflammatory responses, tissue repair, and the modulation of immune functions, showcasing their therapeutic promise 141 . Challenges include difficulties in recruitment due to the evolving clinical landscape, lack of preclinical data, and variations in stem-cell properties. Despite these hurdles, stem-cell therapy, especially considering advancements in organoid technology for better modeling of viral effects, has significant clinical potential 142 . Despite current limitations and technological challenges, the continuous advancement of stem-cell treatment offers optimism in the fight to preserve lives and improve treatment results for individuals with severe COVID-19 infection (Fig. 4 ).
Potential and mechanism of action of mesenchymal stem cell treatment for COVID-19 pneumonia using MSCs, which have immunoregulatory characteristics, can help control the cytokine storm and COVID-19 lung injury. Mesenchymal stromal cells (MSCs) play an important role in a number of processes, including preventing neutrophil infiltration and transforming hyperactivated T cells into regulatory T cells (Tregs). They also promote the production of anti-inflammatory cytokines, such as prostaglandin E2 (PGE2), transforming growth factor beta (TGF), indoleamine 2,3-dioxygenase (IDO), and interleukin 10 (IL-10). Nevertheless, MSCs play a crucial function by stimulating the synthesis of growth factors by endothelial and epithelial cells, which in turn inhibits fibrosis and boosts the infusion of alveolar fluid.
Stem-cell therapy in specific medical fields
Regenerative medicine with stem cells has investigated significant capacity across diverse medical specialties, offering innovative solutions for previously challenging conditions 143 . Patients’ stem cells are harvested for autologous stem-cell treatment. Autologous stem cells that have been cultured are cultivated in the lab before transplantation. These cells have the potential to be categorized into modified and unmodified expanded autologous stem cells. Allogeneic stem cells are classified similarly to autologous stem cells, but they come from healthy donors 18 . Autologous stem cells can be readily acquired and do not cause immunological rejection after infusion. Allogeneic stem cells provide multiple benefits, including the ability to select a donor, availability from different sources, minimal likelihood of causing an immune response, and the convenience of being readily available. Allogeneic MSCs are also immunogenic, indicating that they can trigger an immunological response. These cells can generate a memory response in the immune system under specific circumstances 144 – 146 .
Regenerative medicine can restore, repair, or regenerate impaired tissues or organs by harnessing the unique characteristics of stem cells 147 . This topic includes a range of approaches that seek to leverage the extraordinary capacity of stem cells for medical applications. Although stem cells possess the capacity to undergo self-renewal and differentiate into various distinct cell types, they hold great promise as therapeutic agents against various illnesses and wounds 148 . Regenerative medicine aims to create novel methods to repair damaged tissues caused by disease, injury, or aging using stem cells to restore normal function and structure to damaged organs or tissues 149 . These therapies have great potential to revolutionize medical treatments, particularly in areas where conventional medicine falls short of providing effective remedies or cures 150 .
This emerging field presents a promising avenue for personalized cancer treatments, as researchers have delved into harnessing the unique attributes of stem cells to create innovative strategies for cancer management and potential cures. These investigations signify a significant paradigm shift in oncology, offering a progressive outlook for tailored therapies and potential breakthroughs in cancer treatment 151 , 152 . Stem cell-based cancer treatments are becoming increasingly promising. Because stem cells can locate and target primary and metastatic tumors, and serve as innovative delivery approaches. In preclinical animal models, stem cells modified to express different cytotoxic chemicals consistently reduced tumor size and increased survival 153 , 154 . They have also been used to reduce side effects and improve primary medicinal efficacy by acting as carriers of viruses and nanoparticles. Additionally, stem cells have the potential for utilization in immunotherapy, anticancer drug screening, regenerative medicine, and cancer stem cell-targeted therapy for diverse forms of malignancies, including lung cancer, breast cancer, and osteosarcoma 155 .
Regenerative strategies in orthopedics include advanced osteonecrosis of the hip joint, intervertebral hernias, osteoporosis, targeted joint injuries, cartilage restoration, and bone healing through stem-cell and tissue-engineering methodologies 156 , 157 . Recent investigations have shown innovative approaches, like MSC therapy, platelet-rich plasma (PRP) injections, and biocompatible scaffolds infused with growth factors 158 . These methods aim to optimize cartilage repair and bone regeneration, offering promising outcomes under musculoskeletal conditions 159 , 160 . Research has focused on refining MSC isolation techniques, deciphering the crucial signaling pathways involved in tissue regeneration, and developing bioactive materials that enhance healing 161 .
In the cardiology field, innovative approaches, including stem-cell therapy and bioengineered cardiac patches, are being explored to mend and regenerate impaired heart tissues after cardiac events such as myocardial infarctions 162 . Current research has been focused on different stem-cell types, including iPSCs and cardiac progenitor cells, to regenerate impaired heart muscles and restore cardiac function. Furthermore, research has focused on creating bioengineered cardiac patches using cell-based structures and biomaterials that resemble genuine heart tissue 163 .
In the field of neurology, ongoing investigations have delved into the domain of medicines based on stem cells developed to fight diseases affecting the nervous system, including Parkinson’s and Alzheimer’s 164 , 165 . Studies have focused on using stem cell-derived neurons to replace and regenerate impaired nerve cells 166 . Recent studies have shown that there are numerous varieties of stem cells, including neural stem cells and iPSCs, with the aim of producing functional neurons capable of integrating into damaged neural networks 167 , 168 .
Regenerative medicine in dermatology represents a dynamic frontier of research, particularly concerning stem-cell applications in the skin 169 . Stem cells residing in the skin tissues offer promising avenues for innovative therapeutic strategies that target various dermatological conditions and injuries 170 . Their remarkable regenerative potential holds immense promise for advancing wound healing, addressing burns, and managing skin disorders such as psoriasis and vitiligo 171 , 172 . Additionally, stem cell use in cosmetic dermatology for antiaging treatments and improving skin quality underscores their diverse clinical utility 173 . Researchers have actively explored methods to harness the inherent regenerative abilities of stem cells with the aim of developing tailored and effective therapies for combating skin-related diseases and facilitating cosmetic enhancements. This transformative approach involves tissue engineering techniques utilizing stem cells, biomaterials, and growth factors to create skin substitutes that promote tissue regeneration and repair 174 – 177 .
Due to their potential function, an enormous amount of curiosity about stem cells has persisted in rejuvenating the retina and addressing corneal damage, particularly in diseases such as macular degeneration 178 . Noteworthy studies featured in journals such as ‘Investigative Ophthalmology and Visual Science’ and ‘British Journal of Ophthalmology’ delve into the strides made in utilizing stem cells for ocular regeneration 179 , 180 . Studies have employed stem-cell therapies to restore retinal cells and heal corneal injuries, presenting encouraging pathways for managing vision-related ailments 181 . These studies signify a burgeoning field of ophthalmology research, offering promising prospects for innovative treatments aimed at addressing ocular disorders and enhancing vision 182 .
Stem-cell utilization in oncology, regenerative medicine, and disease therapeutics is an expanding field of research and innovation 151 . Research has focused on leveraging stem cells for targeted cancer therapies and exploring their potential for cellular reprogramming and immune cell modulation to combat tumors 183 . The immunomodulatory potential of stem cells presents a compelling avenue in biomedical research, particularly in addressing autoimmune disorders and graft-versus-host disease (GVHD) and improving transplantation outcomes 184 . Stem cells show a remarkable ability to influence immune cell behavior and function, offering promising prospects for novel therapeutic interventions 185 . This intersection of immunology and stem-cell biology promises not only innovative treatments but also deeper insights into the complex mechanisms governing immune system regulation and dysregulation. This rapidly expanding field has an enormous potential to improve our knowledge of immune-related disorders and provide efficient treatment plans 186 .
Stem-cell utilization in hematology is a dynamic area of scientific inquiry and clinical application in the regenerative medicine field and therapeutic interventions for diseases 187 . Leveraging the potential of stem cells to regenerate is the main goal of research, particularly in HSCs, for transplanting bone marrow and exploring its role in immune cell therapies to combat various blood-related ailments 188 . This growing field represents a promising avenue for innovative treatments, emphasizing the pivotal role of stem cells in revolutionizing hematology by offering potential cures and personalized therapeutic solutions for blood disorders, thereby marking a transformative shift in disease management 189 , 190 .
Stem-cell research offers the potential for addressing illnesses such as inflammatory bowel disease (IBD) 191 , 192 and managing various gastrointestinal disorders 193 . Researchers are investigating stem cell-based approaches to repair gastrointestinal tract injuries, manage ulcers, and alleviate the symptoms of chronic conditions like ulcerative colitis and Crohn’s disease 194 , 195 . Despite ongoing investigations, the clinical application of stem-cell therapies in gastroenterology remains the subject of clinical trials and extensive research, emphasizing the need for further exploration and understanding of their efficacy and safety in treating many immunopathological diseases (Fig. 5 ) 196 .

MSCs inhibit many immunopathological disease conditions, including skin infection, inflammatory bowel disease, and endocrine hormone disorders; they also suppress tumor cells, the aging process, and reproductive infertility.
Ongoing investigations explore the potential of stem cells in restoring lung tissue damaged by diseases like serious respiratory disease or chronic obstructive pulmonary disease (COPD) 197 . Researchers have investigated the capacity of stem cells to restore impaired lung tissue, alleviate COPD symptoms, and target conditions such as idiopathic pulmonary fibrosis 198 . Despite extensive research, the use of stem-cell therapies in pulmonology requires further examination to establish their safety, effectiveness, and long-term effects on respiratory illnesses 199 . Although this emerging field shows promise for future treatment, it requires thorough comprehension and robust clinical validation 200 .
Stem-cell research in reproductive medicine opens new avenues for treating infertility and addressing various reproductive system disorders 201 . Stem cells, whether derived from embryonic, adult, or induced pluripotent sources, hold promise for regenerating and repairing damaged reproductive tissues 202 . This area of study covers various aspects of reproductive health, including the restoration of ovarian function, addressing endometrial issues, and potentially aiding fertility preservation. Research endeavors detailed in publications such as the ‘Journal of Assisted Reproduction and Genetics’ and ‘Fertility and Sterility’, explore the potential of interventions utilizing stem cells to revolutionize infertility treatments and offer new hope to individuals facing reproductive health challenges. These advancements represent a burgeoning field that may reshape the landscape of reproductive medicine and provide innovative solutions for the treatment of infertility and related disorders 201 , 203 .
Stem-cell research in endocrinology presents a promising avenue for managing endocrine disorders such as diabetes by focusing on the generation of insulin-producing cells and regenerating pancreatic tissues 204 . Through various studies documented in journals like ‘Diabetes’ and ‘Endocrine Reviews’, researchers work to create functional beta cells or islet-like structures that can secrete insulin by utilizing the regeneration ability of stem cells 205 . This pioneering field aims to address deficiencies observed in traditional diabetes management by offering cell-based therapies that can potentially restore insulin production and regulate glucose levels 206 . The exploration of stem-cell therapies in endocrinology has heralded a new era of diabetes treatment, offering hope for more effective and sustainable management strategies for this chronic condition 207 .
In dentistry, cutting-edge research has focused on the innovative utilization of stem cells to regenerate crucial dental tissues, including tooth enamel, dentin, and dental pulp 208 . This revolutionary exploration seeks to redefine conventional approaches to dental care by offering transformative treatments for prevalent conditions such as cavities, gum diseases, and dental trauma 209 . Utilizing their unique regenerative stem-cell capacities, scientists aim to generate interventions that induce the natural regeneration and repair of diseased or impaired dental tissues, potentially revolutionizing the oral healthcare landscape 210 , 211 . This promising field of study in dentistry holds the potential to pave the way for novel therapeutic strategies that offer patients improved outcomes and enhanced oral health 212 .
In the domains of trauma and wound healing, intensive research efforts have focused on uncovering the regenerative processes of stem cells to address the complexities of chronic wounds, burns, and traumatic injuries 213 . Stem cells exhibit promising capabilities in fostering tissue regeneration and mitigating scarring by influencing cell differentiation and supporting repair mechanisms in damaged tissues 214 , 215 . This exploration of stem cell-based interventions aims to revolutionize conventional wound care approaches by fostering natural tissue regeneration, accelerating healing processes, and minimizing scarring, thereby offering renewed hope to patients with challenging wounds and traumatic injuries 216 . In the quest for more potent treatment approaches to enhance patient outcomes and accelerate recovery, the potential of stem cells in trauma and wound healing serves as a ray of hope 217 , 218 . Stem-cell regenerative medicine is a dynamic and expansive field, continuously expanding its applications across various medical disciplines to address a wide spectrum of health conditions and diseases 219 (Table 2 ).
Stem-cell therapy is utilized in specific medical fields.
In addition, various types of stimulation have been utilized during stem-cell therapy to enhance differentiation proliferation and improve healing, such as shock wave stimulation 242 . MSCs are increasingly being acknowledged as valuable resources for various orthopedic applications, and radial shock waves have been shown to substantially enhance the development and regrowth of MSCs in a laboratory setting. Furthermore, this type of stimulation safely accelerates cartilage repair in living organisms, suggesting positive results for clinical applications 243 . IR is a type of high-energy radiation that has enough energy to dislodge firmly bound electrons from atoms, leading to the creation of ions. In addition to being a carcinogen, IR is also used as a therapeutic option for patients with cancer. However, there is increasing data showing that extranuclear components, such as mitochondria, play a significant role in the cellular response to IR, and the mitochondrial function of MSCs was observed to be considerably increased after 4 h of exposure to ionizing radiation, as determined by measuring mitochondrial oxygen consumption 244 . Cell proliferation has been induced in many in vitro trials using a modest amount of laser therapy. Osteoblasts, lymphocytes, keratinocytes, and fibroblasts exhibit enhanced proliferation when exposed to laser irradiation 245 . Other types of stimulation include electrical stimulation to enhance stem-cell therapy in nerve regeneration 242 , electrical stimulation to promote cell differentiation and proliferation of fatal neuronal stem cells into neuronal stem cells 246 , and nonpeptide small molecules 247 , in addition to mechanical stimuli such as cyclic stretch, three forces, laminar shear stress, cyclic pressure 248 , and gamma radiation 249 .
Biotechnological advancements in stem-cell research
Stem-cell studies have been significantly promoted by cutting-edge technologies that have revolutionized our understanding and utilization of these versatile cells. This discussion focuses on some of the most impactful biotechnological advancements in stem-cell studies, with a specific focus on exosome-based therapeutics, scRNA-Seq, and the revolutionary CRISPR-Cas9 gene-editing technology 250 – 252 .
Exosome-based therapeutics and stem cells
The new frontier of exosomes produced from stem cell-based therapeutics represents a promising avenue for the field of regenerative medicine 253 . RNAs, signaling molecules, and proteins are bioactive substances encapsulated in exosomes and small vessels secreted by stem cells. These nanovesicles are essential for intercellular interactions and can control a number of cellular functions 254 . Stem cell-derived exosomes exhibit unique properties that modulate immune responses, promote tissue regeneration, and foster repair mechanisms 255 . Harnessing the therapeutic potential of these exosomes holds considerable promise for developing innovative treatments for diverse medical conditions, including inflammatory disorders, neurodegenerative diseases, and tissue injuries 253 , 256 – 258 . Stem cell-derived exosome-based therapies represent a burgeoning frontier in regenerative medicine, providing new opportunities for targeted, minimally invasive therapeutic interventions 259 .
Single-cell RNA sequencing and stem-cell research
Advances in scRNA-seq have allowed investigators to examine stem-cell transcriptomes individually, providing unprecedented insights into cellular heterogeneity and gene expression patterns 13 . This technology has played an essential role in comprehending the dynamics of stem-cell populations during differentiation and disease progression 260 , 261 .
CRISPR-Cas9 technology and gene editing in stem cells
With the advent of CRISPR-Cas9, a new era in gene editing has begun, which enables the precise modifications of stem-cell DNA 14 . Researchers can now edit or introduce specific genes with unprecedented accuracy, facilitating cancer and disease modeling, studying gene function, and developing potential therapeutic interventions 262 , 263 .
CRISPR-based technologies have enabled large-scale functional genomic studies and high-throughput screening of stem cells. That allows researchers to systematically interrogate gene function on a genome-wide scale, uncovering novel regulators of stem-cell fate, pluripotency, and differentiation 264 , 265 .
Beyond traditional CRISPR-Cas9, recent innovations, such as base editing and prime editing, offer enhanced precision in gene editing 266 . These techniques allow the modification of specific nucleotides without causing double-strand breaks, minimizing off-target effects and expanding the possibilities for therapeutic genome editing in stem cells 267 (Fig. 6 ).
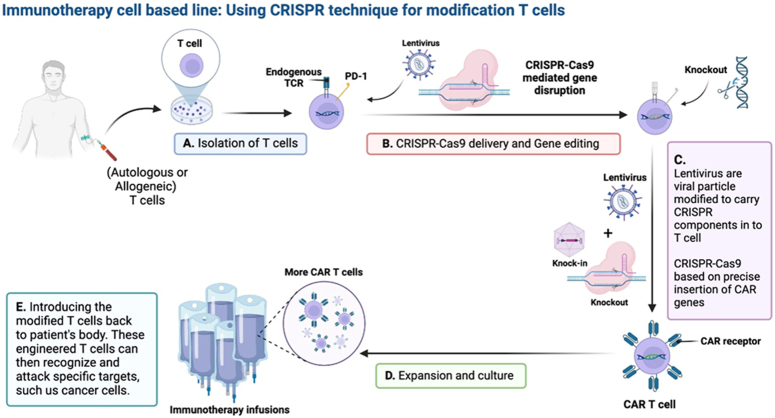
Immunotherapy chimeric antigen receptor (CAR) T-cell therapy can be filled with the help of recent developments in genome editing using CRISPR-Cas9. To enable robust, accurate, and controllable genetic alteration, genome editing techniques are used, such as base and prime editing. In both hematopoietic and non-hematopoietic cancers, T-cells can be circumvented through CRISPR-Cas9-induced multiplex deletion of inhibitory molecules, which enhances CAR T-cell growth and persistence. The use of targeted knock-in techniques during CAR T-cell engineering offers the possibility of producing highly effective and potent cell products. Lentivirus is viral particles modified to carry CRISPR components in T cells, CRISPR-Cas9 based on the precise insertion of CAR genes, more and strong CAR T-cells product engineered using CRISPR-Cas9 to overcome specific histocompatibility hurdles and with improved persistence/antitumor function could greatly improve the production of cellular immunotherapies and the therapeutic durability.
Overall, CRISPR-based gene editing shows great promise for therapeutic applications in stem cell-based regenerative medicine. This opens new avenues for correcting genetic mutations underlying various diseases, generating genetically modified cells for transplantation, and developing personalized cell therapies.
Personalized medicine and stem cells
Stem cells are integral to the advancement of personalized medicine, aligned with the goal of tailoring healthcare to individual characteristics and encompassing genetic, environmental, and lifestyle factors 16 . From a patient’s cells, iPSCs provide a potent platform for building disease models that accurately reflect the person’s genetic background 268 . This capability facilitates in-depth studies of disease mechanisms at the cellular and molecular levels, enabling more precise diagnosis and the establishment of targeted therapeutic strategies 60 . Moreover, modern gene-editing techniques, including CRISPR-Cas9, enable accurate alterations in stem-cell genomes 269 .
This breakthrough allowed the correction of genetic mutations associated with diseases, laying the groundwork for personalized therapies addressing specific genetic alterations in individual cells 270 . In pharmacogenomics, stem cells significantly contribute to the assessment of individual drug responses. Leveraging patient-derived stem cells in pharmacogenomic studies enables researchers to understand the impact of an individual’s genetic composition on their reaction to various medications 271 . This knowledge serves as a guide for formulating personalized treatment plans, minimizing adverse reactions, and enhancing the overall therapeutic outcomes. Moreover, stem cells actively contribute to the identification of personalized biomarkers associated with specific diseases 272 . Differentiating patient-derived stem cells into cell types relevant to the disease makes it easier to identify molecular signatures that can be used as diagnostic indicators. These personalized biomarkers substantially improve the accuracy of disease detection and monitoring, marking a significant step toward more individualized and effective healthcare strategies 273 , 274 .
Stem-cell clinical trials
Stem-cell therapy is witnessing a surge in clinical trials, reflecting a growing interest in translating laboratory findings into viable treatments 275 . Clinical trials involving various stem-cell types are currently underway and include a wide range of health issues 276 . The goal of ongoing trials is to determine whether stem-cell therapies are effective in alleviating symptoms of neurological diseases such as Alzheimer’s, Parkinson’s, and spinal cord injuries 277 (Table 3 ). Researchers are investigating how stem cells might be able to repair damaged neurons, encourage brain regeneration, and lessen the symptoms of these crippling conditions 288 .
Examples of clinical trials with results involved in neurological diseases, cancer, cardiovascular, and Orthopedics, from http://clinicaltrials.gov/ .
Clinical trials in cardiovascular medicine aim to evaluate the use of stem cells, such as progenitor cells and MSCs, for treating conditions like heart failure and ischemic heart disease. These trials explored the regenerative potential of stem cells in repairing impaired cardiac tissues and improving overall cardiac function 289 .
Research is now being conducted on stem cell-based therapeutics for cancer treatment, including studies focusing on HSC transplantation (SCT) to treat hematological malignancies 290 . In addition, researchers have explored potential applications for stem cells in conjunction with traditional cancer therapies in order to enhance therapeutic results and minimize negative consequences 291 . Additionally, clinical trials in orthopedics and musculoskeletal disorders involve the use of stem cells to treat conditions like osteoarthritis and bone defects. MSCs, which are known for their capacity to differentiate into bone and cartilage, are being studied for their regenerative potential in restoring joint and bone health 292 . Furthermore, stem-cell therapies are now under investigation for their potential applications to treat diabetes by replenishing pancreatic beta cells. Clinical trials have investigated the use of stem cell-derived insulin-producing cells as transplants to regulate blood glucose levels in patients with diabetes 293 .
Challenges and ethical considerations
Stem-cell therapy, although showing great promise, faces multiple obstacles and constraints that need to be carefully considered. One prominent challenge is the potential for tumorigenesis, wherein the number of transplanted stem cells may increase uncontrollably, leading to tumor formation 294 , 295 . The security of stem cells can only be ensured by thorough preclinical examinations before they can be used in clinical settings. Additionally, the immune response poses a challenge due to the recipient’s immune system perceiving the transplanted cells as alien, leading to rejection 296 , 297 . The development of strategies to mitigate immune rejection and improve engraftment remains an ongoing challenge.
Furthermore, precisely controlling stem cell development into the desired cell types is a significant challenge 298 . The variability in differentiation protocols and the possibility of off-target consequences raise concerns regarding the reliability and safety of the therapeutic outcomes. Additionally, scalability and cost-effectiveness in the production of sufficient quantities of quality-controlled stem cells for widespread clinical use remain logistic obstacles that must be overcome for the field to attain its full potential 110 , 299 , 300 .
Ethical considerations are central to the discourse surrounding stem-cell therapy, particularly the use of ESCs 301 . Discussions over the moral standing of the early human embryo arose because of the killing of embryos during the extraction of ESCs. Because of these concerns, scientists are looking at alternative sources of pluripotent stem cells, such as iPSCs, which are reprogrammed from adult cells and do not have the same ethical concerns as ESCs. Regulatory frameworks are essential for negotiating the moral challenges presented by different stem cell therapies 302 .
Countries have varying regulations governing the clinical utilization of stem cells, ranging from permissive to restrictive. Achieving a balance between promoting innovation and ensuring patient safety remains a challenge for regulatory bodies 303 . The evolving nature of stem-cell research and therapies necessitates dynamic regulatory frameworks that can be adapted for scientific advancement. Ongoing debates persist in this field, particularly regarding the commercialization of stem-cell therapies. Issues of accessibility, affordability, and equitable distribution of these therapies raise ethical questions.
Moreover, concerns regarding the premature marketing of unproven stem-cell therapies and the need for transparent communication regarding the state of scientific evidence contribute to the ethical complexity of this field 304 . In conclusion, addressing the difficulties and ethical considerations of stem-cell therapy requires a multidisciplinary approach that encompasses rigorous scientific research, transparent communication, and dynamic regulatory frameworks. Realizing the full promise of stem-cell therapies will require a careful balance between ethical responsibility and innovation as the field develops.
Future prospects
With the help of new technologies and the results of continuing research, stem-cell treatment might potentially transform many different areas of medicine. One key direction involves the integration of stem-cell therapy into precision medicine approaches, opening a new chapter in medical history, where customized care based on a person’s genetic composition promises enhanced therapeutic outcomes and reduced side effects. Advances in genomics and the application of patient-specific stem cells are expected to drive this integration. Additionally, future research should focus on refining the immune modulation strategies associated with stem-cell therapies and addressing challenges such as immune rejection and graft-versus-host responses. Innovative approaches, including engineered stem cells and immunomodulatory molecules, aim to enhance compatibility with stem-cell treatment.
The continued evolution of gene-editing tools, including CRISPR-Cas9, will perform a key function in ensuring the precision and safety of stem-cell therapies. This technology enables the modification of specific genes in stem cells, offering avenues for targeted therapeutic interventions and correction of genetic disorders at the cellular level. The synergy between stem-cell therapy and bioengineering has emerged as a significant area of exploration. The integration of stem cells with advanced biomaterials can potentially create functional tissues and organs with improved structural and functional properties. Bioengineered constructs provide innovative solutions for tissue-specific regeneration and transplantation. These key directions underscore the multidimensional nature of future advancements in stem-cell therapy, bringing together precision medicine, immune modulation, gene editing, and bioengineering to propel the field toward transformative developments.
Recent developments in stem-cell therapy have illuminated a path of immense promise and transformative potential for revolutionizing modern medicine. The exploration of stem cells across diverse medical disciplines guided by advancements in science, biotechnology, and clinical trial applications has positioned this field at the forefront of biomedical research. The historical journey from foundational concepts laid by pioneering scientists in the late 19th and early 20th centuries to groundbreaking milestones such as the isolation of ESCs and the discovery of iPSCs underscores a monumental leap in medical science.
The regenerative processes of stem cells, categorized into embryonic, adult, induced pluripotent, and perinatal stem cells, offer unprecedented opportunities for therapeutic interventions. Development, tissue repair, and regeneration are all intricately linked to stem cells due to their remarkable capacity to differentiate into different cell types and self-renew. Their diverse applications include neurodegenerative disorders, cardiovascular ailments, spinal cord injuries, diabetes, and tissue damage, opening novel avenues for treating debilitating conditions. However, as the field advances, the critical challenges and complexities must be addressed. Problems like immunological rejection, tumorigenesis, and the precise manipulation of stem-cell behavior pose hurdles that demand comprehensive exploration and innovative solutions. The landscape of stem-cell therapy is intricate and requires a nuanced understanding of its historical foundations, current realities, and future trajectories.
In collating recent biotechnology advancements, critical trial evaluations, and emerging technologies, this review provides a comprehensive compass for clinicians, researchers, and stakeholders navigating the intricate terrain of stem-cell therapy. Future directions, marked by precision medicine integration, immune modulation strategies, advancements in gene-editing technologies, and synergy with bioengineering, offer a roadmap for the continued evolution of stem-cell therapies.
Resonating with the revolutionary promise of stem-cell therapy not only in the realms of science and medicine but also in the lives of individuals with debilitating diseases and injuries. The journey from conceptualization to practical utilization represents a testament to human ingenuity and the relentless pursuit of improving healthcare. As stem-cell research continues, it holds the promise of reshaping the landscape of medicine, bringing forth a new era in which personalized regenerative therapies can mitigate the impact of a spectrum of medical challenges.
Ethical approval
Not applicable.
Source of funding
No funding was received for this study.
Author contribution
B.M.H.: study design and data analysis; R.K.Y.: writing the paper; G.H.A.: data collection; S.R.A. and R.K.K.: data analysis and interpretation; S.A.M.: study design and writing the paper.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Bashdar Mahmud Hussen and Suhad A. Mustafa.
Data availability statement
All the data are available in the manuscript.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 5 November 2024
Contributor Information
Bashdar M. Hussen, Email: [email protected].
Mohammad Taheri, Email: [email protected].
Raya Kh. Yashooa, Email: [email protected].
Gaylany H. Abdullah, Email: [email protected].
Snur R. Abdullah, Email: [email protected].
Ramiar Kamal Kheder, Email: [email protected].
Suhad A. Mustafa, Email: [email protected].
- 1. Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 2015;17:11–22. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Hansford S, Huntsman DG. Boveri at 100: Theodor Boveri and genetic predisposition to cancer. J Pathol 2014;234:142–145. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Maximow AA. Der lymphozyt als gemeinsame stammzelle der verschiedenen blutelemente in der embryonalen entwicklung und im postfetalen leben der säugetiere. Cellul Ther Transplantat 2009;1:9–13. [ Google Scholar ]
- 5. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292:154–156. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Zhao T, Zhang Z-N, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature 2011;474:212–215. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Lister R, Pelizzola M, Kida YS, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 2011;471:68–73. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–1920. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Diederichs S, Shine KM, Tuan RS. The promise and challenges of stem cell-based therapies for skeletal diseases: stem cell applications in skeletal medicine: potential, cell sources and characteristics, and challenges of clinical translation. Bioessays 2013;35:220–230. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell 2012;148:46–57. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Blau HM, Daley GQ. Stem cells in the treatment of disease. New Engl J Med 2019;380:1748–1760. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. De Los Angeles A, Ferrari F, Xi R, et al. Hallmarks of pluripotency. Nature 2015;525:469–478. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Chen T, Li J, Jia Y, et al. Single-cell sequencing in the field of stem cells. Curr Genomics 2020;21:576–584. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Valenti MT, Serena M, Dalle Carbonare LD, et al. CRISPR/Cas system: an emerging technology in stem cell research. World J Stem Cells 2019;11:937–956. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Han C, Sun X, Liu L, et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int 2016;2016:7653489. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Lattanzi W, Ripoli C, Greco V, et al. Basic and preclinical research for personalized medicine. J Pers Med 2021;11:354. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Kavya ANL, Subramanian S, Ramakrishna S. Therapeutic applications of exosomes in various diseases: a review. Biomat Adv 2022;134:112579. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Mousaei Ghasroldasht M, Seok J, Park H-S, et al. Stem cell therapy: from idea to clinical practice. Int J Mol Sci 2022;23:2850. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Bacakova L, Zarubova J, Travnickova M, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells–a review. Biotechnol Adv 2018;36:1111–1126. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Zhao W, Ji X, Zhang F, et al. Embryonic stem cell markers. Molecules 2012;17:6196–6246. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Chen G, Yin S, Zeng H, et al. Regulation of embryonic stem cell self-renewal. Life (Basel) 2022;12:1151. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natnl Acad Sci 1981;78:7634–7638. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines, derived from human blastocysts. Science 1998;282:1145–1147. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Smith AG. Culture and differentiation of embryonic stem cells. J Tissue Cult Methods 1991;13:89–94. [ Google Scholar ]
- 25. Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 1995;7:862–869. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Wiles MV, Johansson BM. Embryonic stem cell development in a chemically defined medium. Exp Cell Res 1999;247:241–248. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Guest D, Allen W. Expression of cell-surface antigens and embryonic stem cell pluripotency genes in equine blastocysts. Stem Cells Dev 2007;16:789–796. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Saito S, Ugai H, Sawai K, et al. Isolation of embryonic stem‐like cells from equine blastocysts and their differentiation in vitro1. FEBS Lett 2002;531:389–396. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation 2010;122:517–526. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Dvash T, Ben-Yosef D, Eiges R. Human embryonic stem cells as a powerful tool for studying human embryogenesis. Pediatr Res 2006;60:111–117. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. He Q, Li J, Bettiol E, et al. Embryonic stem cells: new possible therapy for degenerative diseases that affect elderly people. J Gerontol A Biol Sci Med Sci 2003;58:M279–M287. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Nichols J, Smith A. The origin and identity of embryonic stem cells. Development 2011;138:3–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell 2011;9:402–411. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Tweedell KS. The adaptability of somatic stem cells: a review. J Stem Cells Regen Med 2017;13:3–13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Vats A, Tolley N, Polak J, et al. Stem cells: sources and applications. Clin Otolaryngol All Sci 2002;27:227–232. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Gurusamy N, Alsayari A, Rajasingh S, et al. Adult stem cells for regenerative therapy. Prog Mol Biol Transl Sci 2018;160:1–22. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol 2006;420:339–361. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Fernández-Susavila H, Bugallo-Casal A, Castillo J, et al. Adult stem cells and induced pluripotent stem cells for stroke treatment. Front Neurol 2019;10:908. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol 2016;2016:6940283. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Franceschetti T, De Bari C. The potential role of adult stem cells in the management of the rheumatic diseases. Ther Adv Musculoskelet Dis 2017;9:165–179. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Cable J, Fuchs E, Weissman I, et al. Adult stem cells and regenerative medicine—a symposium report. Ann N Y Acad Sci 2020;1462:27–36. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Mariano ED, Teixeira MJ, Marie SKN, et al. Adult stem cells in neural repair: current options, limitations and perspectives. World J Stem Cells 2015;7:477. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Tan KY, Kim FS, Wagers AJ, et al. Hematopoietic stem cells and somatic stem cells. Hematopoietic Stem Cell Biol 2010:57–92. [ Google Scholar ]
- 45. Lee JY, Hong SH. Hematopoietic stem cells and their roles in tissue regeneration. Int J Stem Cells 2020;13:1–12. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Dessie G, Derbew Molla M, Shibabaw T, et al. Role of stem-cell transplantation in leukemia treatment. Stem Cells Clon Adv Appl 2020;13:67–77. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Swart JF, Delemarre EM, Van Wijk F, et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol 2017;13:244–256. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Hatzimichael E, Tuthill M. Hematopoietic stem cell transplantation. Stem Cells Cloning 2010;3:105–117. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Torre Pdl, Flores AI. Current status and future prospects of perinatal stem cells. Genes (Basel) 2020;12:6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Chen P-M, Yen M-L, Liu K-J, et al. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci 2011;18:49. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Deus IA, Mano JF, Custodio CA. Perinatal tissues and cells in tissue engineering and regenerative medicine. Acta Biomater 2020;110:1–14. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Brown PT, Handorf AM, Jeon WB, et al. Stem cell-based tissue engineering approaches for musculoskeletal regeneration. Curr Pharm Des 2013;19:3429–3445. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Paris F, Marrazzo P, Pizzuti V, et al. Characterization of perinatal stem cell spheroids for the development of cell therapy strategy. Bioengineering 2023;10:189. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Li Z, Han ZC. Introduction of perinatal tissue-derived stem cells. Perinatal Stem Cells Biol Manufacturing Transl Med 2019:1–7. [ Google Scholar ]
- 55. Omole AE, Fakoya AOJ. Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ 2018;6:e4370. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Kim JB, Zaehres H, Wu G, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 2008;454:646–650. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Kunisato A, Wakatsuki M, Kodama Y, et al. Generation of induced pluripotent stem cells by efficient reprogramming of adult bone marrow cells. Stem Cells Dev 2010;19:229–238. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Ye L, Swingen C, Zhang J. Induced pluripotent stem cells and their potential for basic and clinical sciences. Curr Cardiol Rev 2013;9:63–72. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Bilic J, Belmonte JCI. Concise review: induced pluripotent stem cells versus embryonic stem cells: close enough or yet too far apart? Stem Cells 2011;30:33–41. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Park S, Gwon Y, Khan SA, et al. Engineering considerations of iPSC-based personalized medicine. Biomater Res 2023;27:67. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Shi Y, Inoue H, Wu JC, et al. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov 2017;16:115–130. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Doss MX, Sachinidis A. Current challenges of iPSC-based disease modeling and therapeutic implications. Cells 2019;8:403. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Karami Z, Moradi S, Eidi A, et al. Induced pluripotent stem cells: generation methods and a new perspective in COVID-19 research. Front Cell Dev Biol 2023;10:1050856. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Moradi S, Mahdizadeh H, Šarić T, et al. Research and therapy with induced pluripotent stem cells (iPSCs): social, legal, and ethical considerations. Stem Cell Res Ther 2019;10:1–13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Gao F, Chiu S, Motan D, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 2016;7:e2062. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Mili B, Choudhary OP. Advancements and mechanisms of stem cell-based therapies for spinal cord injury in animals. Int J Surg 2024;110:6182–6197. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Kode JA, Mukherjee S, Joglekar MV, et al. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009;11:377–391. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif 2020;53:e12712. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med 2008;26:711–715. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer 2018;6:1–14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Lee K-H, Tseng W-C, Yang C-Y, et al. The anti-inflammatory, anti-oxidative, and anti-apoptotic benefits of stem cells in acute ischemic kidney injury. Int J Mol Sci 2019;20:3529. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Kristensen DM, Kalisz M, Nielsen JH. Cytokine signalling in embryonic stem cells. APMIS 2005;113(11‐12):756–772. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Schnabel LV, Abratte CM, Schimenti JC, et al. Induced pluripotent stem cells have similar immunogenic and more potent immunomodulatory properties compared with bone marrow-derived stromal cells in vitro. Regen Med 2014;9:621–635. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Bernard O, Jeny F, Uzunhan Y, et al. Mesenchymal stem cells reduce hypoxia-induced apoptosis in alveolar epithelial cells by modulating HIF and ROS hypoxic signaling. Am J Physiol Lung Cell Mol Physiol 2018;314:L360–L371. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. New Engl J Med 2007;357:1450–1451. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Pan S, Zhao X, Wang X, et al. Sfrp1 attenuates TAC-induced cardiac dysfunction by inhibiting Wnt signaling pathway-mediated myocardial apoptosis in mice. Lipids Health Dis 2018;17:1–6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Najar M, Martel-Pelletier J, Pelletier J-P, et al. Mesenchymal stromal cell immunology for efficient and safe treatment of osteoarthritis. Front Cell Dev Biol 2020;8:567813. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Chow L, Johnson V, Impastato R, et al. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl Med 2020;9:235–249. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010;28:2229–2238. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Han Y, Li X, Zhang Y, et al. Mesenchymal stem cells for regenerative medicine. Cells 2019;8:886. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Nat Rev Bioeng 2023;1:608–609. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. DiMarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol 2013;4:52511. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Shafiee A, Patel J, Lee JS, et al. Mesenchymal stem/stromal cells enhance engraftment, vasculogenic and pro-angiogenic activities of endothelial colony forming cells in immunocompetent hosts. Sci Rep 2017;7:13558. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Matthay MA, Thompson BT, Read EJ, et al. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 2010;138:965–972. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Sadeghi S, Soudi S, Shafiee A, et al. Mesenchymal stem cell therapies for COVID-19: current status and mechanism of action. Life Sci 2020;262:118493. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Fleifel D, Rahmoon MA, AlOkda A, et al. Recent advances in stem cells therapy: a focus on cancer, Parkinson’s and Alzheimer’s. J Genetic Engineer Biotechnol 2018;16:427–432. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev 2008;60:199–214. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Aly RM. Current state of stem cell-based therapies: an overview. Stem Cell Investig 2020;7:8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Xing W, Yang J, Zheng Y, et al. The role of the notch signaling pathway in the differentiation of human umbilical cord-derived mesenchymal stem cells. Front Biosc 2024;29:74. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Fox V, Gokhale PJ, Walsh JR, et al. Cell-cell signaling through NOTCH regulates human embryonic stem cell proliferation. Stem Cells 2008;26:715–723. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Noisa P, Lund C, Kanduri K, et al. Notch signaling regulates the differentiation of neural crest from human pluripotent stem cells. J Cell Sci 2014;127:2083–2094. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Benveniste P, Serra P, Dervovic D, et al. Notch signals are required for in vitro but not in vivo maintenance of human hematopoietic stem cells and delay the appearance of multipotent progenitors. Blood 2014;123:1167–1177. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Dong C, Wang X, Sun L, et al. ATM modulates subventricular zone neural stem cell maintenance and senescence through Notch signaling pathway. Stem Cell Res 2022;58:102618. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Sancho R, Cremona CA, Behrens A. Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO Rep 2015;16:571–581. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Jafari M, Ghadami E, Dadkhah T, et al. PI3k/AKT signaling pathway: erythropoiesis and beyond. J Cell Physiol 2019;234:2373–2385. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Liu C, Peng G, Jing N. TGF-β signaling pathway in early mouse development and embryonic stem cells. Acta Biochim Biophys Sin (Shanghai) 2018;50:68–73. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Kaltschmidt C, Greiner JF, Kaltschmidt B. The transcription factor NF-κB in stem cells and development. Cells 2021;10:2042. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Lyssiotis CA, Lairson LL, Boitano AE, et al. Chemical control of stem cell fate and developmental potential. Angewandte Chemie Int Ed 2011;50:200–242. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Eom Y-S, Park J-H, Kim T-H. Recent advances in stem cell differentiation control using drug delivery systems based on porous functional materials. J Funct Biomater 2023;14:483. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Donowitz M, Turner JR, Verkman AS, et al. Current and potential future applications of human stem cell models in drug development. J Clin Invest 2020;130:3342–3344. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol 2010;28:1079–1088. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Ankam S, Rovini A, Baheti S, et al. DNA methylation patterns in human iPSC-derived sensory neuronal differentiation. Epigenetics 2019;14:927–937. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Ratajczak MZ, Jadczyk T, Pedziwiatr D, et al. New advances in stem cell research: practical implications for regenerative medicine. Pol Arch Med Wewn 2014;124:417–426. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Rodríguez-Fuentes DE, Fernández-Garza LE, Samia-Meza JA, et al. Mesenchymal stem cells current clinical applications: a systematic review. Arch Med Res 2021;52:93–101. [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. Labusca L, Herea DD, Mashayekhi K. Stem cells as delivery vehicles for regenerative medicine-challenges and perspectives. World J Stem Cells 2018;10:43–56. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Anderson JD. Advances in stem cell immunotherapy. Stem Cells 2023;41:307–309. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 107. Sharma SK. Stem cell transplant for autoimmune diseases. Basics of Hematopoietic Stem Cell Transplant. Springer; 2023:247–258. [ Google Scholar ]
- 108. Sakowski SA, Chen KS. Stem cell therapy for central nervous system disorders: metabolic interactions between transplanted cells and local microenvironments. Neurobiol Dis 2022;173:105842. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Rahman MM, Islam MR, Islam MT, et al. Stem cell transplantation therapy and neurological disorders: current status and future perspectives. Biology 2022;11:147. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 110. Mahmud S, Alam S, Emon NU, et al. Opportunities and challenges in stem cell therapy in cardiovascular diseases: position standing in 2022. Saudi Pharmaceut J 2022;30:1360–1371. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 111. Povsic TJ, Gersh BJ. Stem cells in cardiovascular diseases: 30,000-foot view. Cells 2021;10:600. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Maniar H, Tawari A, Suk M, et al. The current role of stem cells in orthopaedic surgery. Malays Orthop J 2015;9:1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 113. Akpancar S, Tatar O, Turgut H, et al. The current perspectives of stem cell therapy in orthopedic surgery. Arch Trauma Res 2016;5:e37976. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 114. Malekpour K, Hazrati A, Zahar M, et al. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rev Rep 2022;18:933–951. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 115. Xie X, Yao H, Han X, et al. Therapeutic use of red blood cells and platelets derived from human cord blood stem cells. Stem Cells Transl Med 2021;10(S2):S48–S53. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 116. Hamad FRB, Rahat N, Shankar K, et al. Efficacy of stem cell application in diabetes mellitus: promising future therapy for diabetes and its complications. Cureus 2021;13:e13563. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 117. Mathur A, Taurin S, Alshammary S. The safety and efficacy of mesenchymal stem cells in the treatment of type 2 diabetes-a literature review. Diab Metabol Syndr Obes 2023;16:769–777. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 118. Bagher Z, Azami M, Ebrahimi-Barough S, et al. Differentiation of Wharton’s jelly-derived mesenchymal stem cells into motor neuron-like cells on three-dimensional collagen-grafted nanofibers. Mol Neurobiol 2016;53:2397–2408. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Rincon‐Benavides MA, Mendonca NC, Cuellar‐Gaviria TZ, et al. Engineered vasculogenic extracellular vesicles drive nonviral direct conversions of human dermal fibroblasts into induced endothelial cells and improve wound closure. Adv Therap 2023;6:2200197. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 120. Wang Z, Yang H, Xu X, et al. Ion elemental-optimized layered double hydroxide nanoparticles promote chondrogenic differentiation and intervertebral disc regeneration of mesenchymal stem cells through focal adhesion signaling pathway. Bioactive Materials 2023;22:75–90. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 121. Mo H, Kim J, Kim JY, et al. Intranasal administration of induced pluripotent stem cell-derived cortical neural stem cell-secretome as a treatment option for Alzheimer’s disease. Transl Neurodegener 2023;12:50. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 122. Zhou Z, Shi B, Xu Y, et al. Neural stem/progenitor cell therapy for Alzheimer disease in preclinical rodent models: a systematic review and meta-analysis. Stem Cell Res Ther 2023;14:3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 123. Hamedi H, Ghorbanian SH, Mirzaeian L, et al. Intravenous transplantation of adipose-derived mesenchymal stem cells promoted the production of dopaminergic neurons and improved spatial memory in a rat model of Parkinson’s disease. Cell J 2023;25:317–326. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 124. Mendes-Pinheiro B, Campos J, Marote A, et al. Treating Parkinson’s disease with human bone marrow mesenchymal stem cell secretome: a translational investigation using human brain organoids and different routes of in vivo administration. Cells 2023;12:2565. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 125. Vo QD, Saito Y, Nakamura K, et al. Induced pluripotent stem cell-derived cardiomyocytes therapy for ischemic heart disease in animal model: a meta-analysis. Int J Mol Sci 2024;25:987. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 126. Zhang J, Li J, Qu X, et al. Development of a thick and functional human adipose-derived stem cell tissue sheet for myocardial infarction repair in rat hearts. Stem Cell Res Ther 2023;14:380. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 127. Freitag J, Bates D, Wickham J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med 2019;14:213–230. [ DOI ] [ PubMed ] [ Google Scholar ]
- 128. Shima T, Takigawa K, Utsumi S, et al. Outcomes of allogeneic stem cell transplantation for patients with hematologic diseases ≥60 years old. Blood Cell Ther 2023;6:30–41. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 129. Ding L, Han D-M, Zheng X-L, et al. A study of human leukocyte antigen-haploidentical hematopoietic stem cells transplantation combined with allogenic mesenchymal stem cell infusion for treatment of severe aplastic anemia in pediatric and adolescent patients. Stem Cells Transl Med 2020;10:291–302. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 130. Zang L, Li Y, Hao H, et al. Efficacy of umbilical cord-derived mesenchymal stem cells in the treatment of type 2 diabetes assessed by retrospective continuous glucose monitoring. Stem Cells Transl Med 2023;12:775–782. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 131. Rezaeian A, Khatami F, Heidari Keshel S, et al. The effect of mesenchymal stem cells-derived exosomes on the prostate, bladder, and renal cancer cell lines. Sci Rep 2022;12:20924. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 132. Sun L, Xu Y, Zhang X, et al. Mesenchymal stem cells functionalized sonodynamic treatment for improving therapeutic efficacy and compliance of orthotopic oral cancer. Adv Mat 2020;32:2005295. [ DOI ] [ PubMed ] [ Google Scholar ]
- 133. Hoseinzadeh A, Mahmoudi M, Rafatpanah H, et al. A new generation of mesenchymal stromal/stem cells differentially trained by immunoregulatory probiotics in a lupus microenvironment. Stem Cell Res Ther 2023;14:358. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 134. Gong Z, He Y, Zhou M, et al. Ultrasound imaging tracking of mesenchymal stem cells intracellularly labeled with biosynthetic gas vesicles for treatment of rheumatoid arthritis. Theranostics 2022;12:2370–2382. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 135. Feng G, Shi L, Huang T, et al. Human umbilical cord mesenchymal stromal cell treatment of severe COVID-19 patients: a 3-month follow-up study following hospital discharge. Stem Cells Dev 2021;30:773–781. [ DOI ] [ PubMed ] [ Google Scholar ]
- 136. Dilogo IH, Aditianingsih D, Sugiarto A, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med 2021;10:1279–1287. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 137. Marfe G, Perna S, Shukla AK. Effectiveness of COVID‑19 vaccines and their challenges. Exp Ther Med 2021;22:1–19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 138. Chen J, Hu C, Chen L, et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19 treatment. Engineering 2020;6:1153–1161. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 139. Kheder RK, Darweesh O, Hussen BM, et al. Mesenchymal stromal cells (MSCs) as a therapeutic agent of inflammatory disease and infectious COVID-19 virus: live or dead mesenchymal? Mol Biol Rep 2024;51:295. [ DOI ] [ PubMed ] [ Google Scholar ]
- 140. Shu L, Niu C, Li R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther 2020;11:1–11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 141. Xie Q, Liu R, Jiang J, et al. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res Ther 2020;11:1–13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 142. Li S, Zhu H, Zhao M, et al. When stem cells meet COVID-19: recent advances, challenges and future perspectives. Stem Cell Res Ther 2022;13:1–16. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 143. Zakrzewski W, Dobrzyński M, Szymonowicz M, et al. Stem cells: past, present, and future. Stem Cell Res Ther 2019;10:1–22. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 144. Zangi L, Margalit R, Reich-Zeliger S, et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009;27:2865–2874. [ DOI ] [ PubMed ] [ Google Scholar ]
- 145. Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006;108:2114–2120. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 146. Schu S, Nosov M, O’Flynn L, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med 2012;16:2094–2103. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 147. Kwon SG, Kwon YW, Lee TW, et al. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater Res 2018;22:36. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 148. Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol 2016;17:194–200. [ DOI ] [ PubMed ] [ Google Scholar ]
- 149. Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med 2008;3:1–5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 150. Atala A. Regenerative medicine strategies. J Pediatr Surg 2012;47:17–28. [ DOI ] [ PubMed ] [ Google Scholar ]
- 151. Mansouri V, Beheshtizadeh N, Gharibshahian M, et al. Recent advances in regenerative medicine strategies for cancer treatment. Biomed Pharmacother 2021;141:111875. [ DOI ] [ PubMed ] [ Google Scholar ]
- 152. Derks LL, van Boxtel R. Stem cell mutations, associated cancer risk, and consequences for regenerative medicine. Cell Stem Cell 2023;30:1421–1433. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 153. Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natnl Acad Sci 2000;97:12846–12851. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 154. Aboody KS, Najbauer J, Metz MZ, et al. Neural stem cell–mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med 2013;5:184ra59–ra59. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 155. Zhang CL, Huang T, Wu BL, et al. Stem cells in cancer therapy: opportunities and challenges. Oncotarget 2017;8:75756–75766. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 156. Evans CH. Advances in regenerative orthopedics. Mayo Clin Proc 2013;88:1323–1339. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 157. Zhao J, Meng H, Liao S, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells in early traumatic osteonecrosis of the femoral head. J Orthop Transl 2022;37:126–142. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 158. Masoudi EA, Ribas J, Kaushik G, et al. Platelet-rich blood derivatives for stem cell-based tissue engineering and regeneration. Curr Stem Cell Rep 2016;2:33–42. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 159. Sundelacruz S, Kaplan DL. Stem cell-and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol. Elsevier; 2009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 160. Andia I, Maffulli N. New biotechnologies for musculoskeletal injuries. Surgeon 2019;17:244–255. [ DOI ] [ PubMed ] [ Google Scholar ]
- 161. Dzobo K, Thomford NE, Senthebane DA, et al. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int 2018;2018:2495848. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 162. Madonna R, Van Laake LW, Botker HE, et al. ESC working group on cellular biology of the heart: tissue engineering and cell-based therapies for cardiac repair in ischemic heart disease and heart failure. Cardiovasc Res 2019;115:488–500. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 163. Arjmand B, Abedi M, Arabi M, et al. Regenerative medicine for the treatment of ischemic heart disease; status and future perspectives. Front Cell Dev Biol 2021;9:704903. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 164. Kim SU, Lee HJ, Kim YB. Neural stem cell‐based treatment for neurodegenerative diseases. Neuropathology 2013;33:491–504. [ DOI ] [ PubMed ] [ Google Scholar ]
- 165. Yashooa RK, Nabi AQ. The miR-146a-5p and miR-125b-5p levels as biomarkers for early prediction of Alzheimer’s disease. Human Gene 2022;34:201129. [ Google Scholar ]
- 166. Sun Y, Feng L, Liang L, et al. Neuronal cell-based medicines from pluripotent stem cells: development, production, and preclinical assessment. Stem Cells Transl Med 2021;10(s2):S31–S40. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 167. Isaković J, Šerer K, Barišić B, et al. Mesenchymal stem cell therapy for neurological disorders: the light or the dark side of the force? Front Bioeng Biotechnol 2023;11:1139359. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 168. Inoue M, Yamaguchi R, He CCJ, et al. Current status and prospects of regenerative medicine for spinal cord injury using human induced pluripotent stem cells: a review. Stem Cell Investig 2023;10:6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 169. Dieckmann C, Renner R, Milkova L, et al. Regenerative medicine in dermatology: biomaterials, tissue engineering, stem cells, gene transfer and beyond. Exp Dermatol 2010;19:697–706. [ DOI ] [ PubMed ] [ Google Scholar ]
- 170. Ojeh N, Pastar I, Tomic-Canic M, et al. Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci 2015;16:25476–25501. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 171. Bellei B, Papaccio F, Picardo M. Regenerative medicine-based treatment for vitiligo: an overview. Biomedicines 2022;10:2744. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 172. Paganelli A, Tarentini E, Benassi L, et al. Mesenchymal stem cells for the treatment of psoriasis: a comprehensive review. Clin Exp Dermatol 2020;45:824–830. [ DOI ] [ PubMed ] [ Google Scholar ]
- 173. Chou Y, Alfarafisa NM, Ikezawa M, et al. Progress in the development of stem cell-derived cell-free therapies for skin aging. Clin Cosmet Investig Dermatol 2023;16:3383–3406. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 174. Davies O, Williams S, Goldie K. The therapeutic and commercial landscape of stem cell vesicles in regenerative dermatology. J Controlled Release 2023;353:1096–1106. [ DOI ] [ PubMed ] [ Google Scholar ]
- 175. Nilforoushzadeh MA, Amirkhani MA, Seirafianpour F, et al. Regenerative medicine in dermatology. J Skin Stem Cell 2022;9. [ Google Scholar ]
- 176. Golchin A. Stem cell technology and skin disorders: from stem cell biology to clinical applications. Stem Cell Rev Rep 2022;18:1881–1882. [ DOI ] [ PubMed ] [ Google Scholar ]
- 177. Khandpur S, Gupta S, Gunaabalaji D. Stem cell therapy in dermatology. Indian J Dermatol Venereol Leprol 2021;87:753–767. [ DOI ] [ PubMed ] [ Google Scholar ]
- 178. Dhamodaran K, Subramani M, Ponnalagu M, et al. Ocular stem cells: a status update!. Stem Cell Res Ther 2014;5:1–12. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 179. Tomczak W, Winkler-Lach W, Tomczyk-Socha M, et al. Advancements in ocular regenerative therapies. Biology 2023;12:737. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 180. Sahle FF, Kim S, Niloy KK, et al. Nanotechnology in regenerative ophthalmology. Adv Drug Del Rev 2019;148:290–307. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 181. Fortress AM, Miyagishima KJ, Reed AA, et al. Stem cell sources and characterization in the development of cell-based products for treating retinal disease: an NEI Town Hall report. BioMed Central 2023;14:53. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 182. Van Gelder RN, Chiang MF, Dyer MA, et al. Regenerative and restorative medicine for eye disease. Nat Med 2022;28:1149–1156. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 183. Zimmerlin L, Park TS, Zambidis ET, et al. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 2013;95:2235–2245. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 184. Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol 2006;34:389–396. [ DOI ] [ PubMed ] [ Google Scholar ]
- 185. Petrus-Reurer S, Romano M, Howlett S, et al. Immunological considerations and challenges for regenerative cellular therapies. Communications Biol 2021;4:798. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 186. Preynat-Seauve O, Krause K-H. Stem cell sources for regenerative medicine: the immunological point of view. Seminars in immunopathology. Springer; 2011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 187. Eskew W. Regenerative medicine: an analysis of origins, trends and potential therapeutic applications, with a focus on hematopoietic stem cells. 2018.
- 188. Skulimowska I, Sosniak J, Gonka M, et al. The biology of hematopoietic stem cells and its clinical implications. FEBS J 2022;289:7740–7759. [ DOI ] [ PubMed ] [ Google Scholar ]
- 189. Montserrat-Vazquez S, Ali NJ, Matteini F, et al. Transplanting rejuvenated blood stem cells extends lifespan of aged immunocompromised mice. NPJ Regen Med 2022;7:78. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 190. Imran SA, M. Hamizul MHA, Khairul Bariah AAN, et al. Regenerative medicine therapy in Malaysia: an update. Front Bioeng Biotechnol 2022;10:789644. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 191. Shimizu H, Suzuki K, Watanabe M, et al. Stem cell-based therapy for inflammatory bowel disease. Intest Res 2019;17:311–316. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 192. Okamoto R, Mizutani T, Shimizu H. Development and application of regenerative medicine in inflammatory bowel disease. Digestion 2023;104:24–29. [ DOI ] [ PubMed ] [ Google Scholar ]
- 193. Yan L, Cai C, Li J, et al. Present status and perspectives of stem cell-based therapies for gastrointestinal diseases. Stem Cell Rev Rep 2009;5:278–282. [ DOI ] [ PubMed ] [ Google Scholar ]
- 194. Peterson J, Pasricha PJ. Regenerative medicine and the gut. Gastroenterology 2011;141:1162–6.e2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 195. Kanetaka K, Eguchi S. Regenerative medicine for the upper gastrointestinal tract. Regen Ther 2020;15:129–137. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 196. Collier CA, Mendiondo C, Raghavan S. Tissue engineering of the gastrointestinal tract: the historic path to translation. J Biol Eng 2022;16:1–12. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 197. Kokturk N, Yildirim F, Gülhan PY, et al. Stem cell therapy in chronic obstructive pulmonary disease. How far is it to the clinic? Am J Stem Cells 2018;7:56. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 198. Adamič N, Vengust M. Regenerative medicine in lung diseases: a systematic review. Front Vet Sci 2023;10:1115708. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 199. Sun Z, Li F, Zhou X, et al. Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials. J Thorac Dis 2018;10:1084. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 200. Singh PV, Singh PV, Anjankar A, et al. Harnessing the therapeutic potential of stem cells in the management of chronic obstructive pulmonary disease: a comprehensive review. Cureus 2023;15:e44498. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 201. Wu J-X, Xia T, She L-P, et al. Stem cell therapies for human infertility: advantages and challenges. Cell Transplant 2022;31:09636897221083252. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 202. Wang J, Liu C, Fujino M, et al. Stem cells as a resource for treatment of infertility-related diseases. Curr Mol Med 2019;19:539–546. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 203. Vassena R, Eguizabal C, Heindryckx B, et al. Stem cells in reproductive medicine: ready for the patient? Hum Reprod 2015;30:2014–2021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 204. Rahim F, Arjmand B, Shirbandi K, et al. Stem cell therapy for patients with diabetes: a systematic review and meta-analysis of metabolomics-based risks and benefits. Stem Cell Investig 2018;5:40. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 205. Brovkina O, Dashinimaev E. Advances and complications of regenerative medicine in diabetes therapy. PeerJ 2020;8:e9746. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 206. Bouwens L, Houbracken I, Mfopou JK. The use of stem cells for pancreatic regeneration in diabetes mellitus. Nat Rev Endocrinol 2013;9:598–606. [ DOI ] [ PubMed ] [ Google Scholar ]
- 207. Li Y, He C, Liu R, et al. Stem cells therapy for diabetes: from past to future. Cytotherapy 2023;25:1125–1138. [ DOI ] [ PubMed ] [ Google Scholar ]
- 208. Soudi A, Yazdanian M, Ranjbar R, et al. Role and application of stem cells in dental regeneration: a comprehensive overview. EXCLI J 2021;20:454. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 209. Thalakiriyawa DS, Dissanayaka WL. Advances in regenerative dentistry approaches: an update. Int Dent J 2023;74:25–34. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 210. Smojver I, Katalinić I, Bjelica R, et al. Mesenchymal stem cells based treatment in dental medicine: a narrative review. Int J Mol Sci 2022;23:1662. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 211. Song W-P, Jin L-Y, Zhu M-D, et al. Clinical trials using dental stem cells: 2022 update. World J Stem Cells 2023;15:31. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 212. Xiao L, Nasu M. From regenerative dentistry to regenerative medicine: progress, challenges, and potential applications of oral stem cells. Stem Cells Clon Adv Applicat 2014;7:89–99. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 213. Kolimi P, Narala S, Nyavanandi D, et al. Innovative treatment strategies to accelerate wound healing: trajectory and recent advancements. Cells 2022;11:2439. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 214. El-Sayed ME, Atwa A, Sofy AR, et al. Mesenchymal stem cell transplantation in burn wound healing: uncovering the mechanisms of local regeneration and tissue repair. Histochem Cell Biol 2024;161:165–181. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 215. Abusalah MAH, Priyanka, Abd Rahman ENSE, et al. Evolving trends in stem cell therapy: an emerging and promising approach against various diseases. Int J Surg. 9900:10.1097/JS9.0000000000001948.
- 216. Fadilah NIM, Jailani MSMAK, Hisham MAIB, et al. Cell secretomes for wound healing and tissue regeneration: Next generation acellular based tissue engineered products. J Tissue Eng 2022;13:20417314221114273. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 217. Zeng N, Chen H, Wu Y, et al. Adipose stem cell-based treatments for wound healing. Front Cell Dev Biol 2022;9:821652. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 218. Urao N, Liu J, Takahashi K, et al. Hematopoietic stem cells in wound healing response. Adv Wound Care 2022;11:598–621. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 219. Kanji S, Das H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediators Inflamm 2017;2017:5217967. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 220. Shi Y, Wang S, Zhang W, et al. Bone marrow mesenchymal stem cells facilitate diabetic wound healing through the restoration of epidermal cell autophagy via the HIF-1α/TGF-β1/SMAD pathway. Stem Cell Res Ther 2022;13:1–16. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 221. Lee YI, Kim S, Kim J, et al. Randomized controlled study for the anti‐aging effect of human adipocyte‐derived mesenchymal stem cell media combined with niacinamide after laser therapy. J Cosmet Dermatol 2021;20:1774–1781. [ DOI ] [ PubMed ] [ Google Scholar ]
- 222. Ma Y, Hao X, Zhang S, et al. The in vitro and in vivo effects of human umbilical cord mesenchymal stem cells on the growth of breast cancer cells. Breast Cancer Res Treat 2012;133:473–485. [ DOI ] [ PubMed ] [ Google Scholar ]
- 223. Li X, Wu F. Mesenchymal stem cell-derived extracellular vesicles transfer miR-598 to inhibit the growth and metastasis of non-small-cell lung cancer by targeting THBS2. Cell Death Discov 2023;9:3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 224. Qiao B, Shui W, Cai L, et al. Human mesenchymal stem cells as delivery of osteoprotegerin gene: homing and therapeutic effect for osteosarcoma. Drug Des Dev Ther 2015;9:969–976. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 225. Bastos R, Mathias M, Andrade R, et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc 2020;28:1989–1999. [ DOI ] [ PubMed ] [ Google Scholar ]
- 226. Marfia G, Navone SE, Di Vito C, et al. Gene expression profile analysis of human mesenchymal stem cells from herniated and degenerated intervertebral discs reveals different expression of osteopontin. Stem Cells Dev 2015;24:320–328. [ DOI ] [ PubMed ] [ Google Scholar ]
- 227. Jiang M, Wang X, Liu H, et al. Bone formation in adipose‐derived stem cells isolated from elderly patients with osteoporosis: a preliminary study. Cell Biol Int 2014;38:97–105. [ DOI ] [ PubMed ] [ Google Scholar ]
- 228. Li N, Huang R, Zhang X, et al. Stem cells cardiac patch from decellularized umbilical artery improved heart function after myocardium infarction. Bio-Med Mater Eng 2017;28(s1):S87–S94. [ DOI ] [ PubMed ] [ Google Scholar ]
- 229. Zhang Q, Wu Hh, Wang Y, et al. Neural stem cell transplantation decreases neuroinflammation in a transgenic mouse model of Alzheimer’s disease. J Neurochem 2016;136:815–825. [ DOI ] [ PubMed ] [ Google Scholar ]
- 230. Kim C-H, Lim C-Y, Lee J-H, et al. Human embryonic stem cells-derived mesenchymal stem cells reduce the symptom of psoriasis in imiquimod-induced skin model. Tissue Eng Regenerative Med 2019;16:93–102. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 231. Zhu L, Lin X, Zhi L, et al. Mesenchymal stem cells promote human melanocytes proliferation and resistance to apoptosis through PTEN pathway in vitiligo. Stem Cell Res Ther 2020;11:1–14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 232. Møller‐Hansen M. Mesenchymal stem cell therapy in aqueous deficient dry eye disease. Wiley Online Library; 2023. [ DOI ] [ PubMed ] [ Google Scholar ]
- 233. Macías-Sánchez MdM, Morata-Tarifa C, Cuende N, et al. Mesenchymal stromal cells for treating steroid-resistant acute and chronic graft versus host disease: A multicenter compassionate use experience. Stem Cells Transl Med 2022;11:343–355. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 234. Lu D, Gong X, Guo X, et al. Therapeutic effects of hematopoietic stem cell derived from gene-edited mice on β654-thalassemia. Stem Cells 2023;42:278–289. [ DOI ] [ PubMed ] [ Google Scholar ]
- 235. Lightner AL, Reese JS, Ream J, et al. A phase IB/IIA study of ex vivo expanded allogeneic bone marrow–derived mesenchymal stem cells for the treatment of rectovaginal fistulizing Crohn’s disease. Surgery 2024;175:242–249. [ DOI ] [ PubMed ] [ Google Scholar ]
- 236. Zhang X, Hu T, Yu X, et al. Human umbilical cord mesenchymal stem cells improve lung function in chronic obstructive pulmonary disease rat model through regulating lung microbiota. Stem Cells 2024;42:346–359; sxae007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 237. Liao W, Tang X, Li X, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on tubal factor infertility using a chronic salpingitis murine model. Arch Gynecol Obstet 2019;300:421–429. [ DOI ] [ PubMed ] [ Google Scholar ]
- 238. Edessy M, Hosni HN, Shady Y, et al. Autologous stem cells therapy, the first baby of idiopathic premature ovarian failure. Acta Medica Int 2016;3:19–23. [ Google Scholar ]
- 239. Aierken A, Li B, Liu P, et al. Melatonin treatment improves human umbilical cord mesenchymal stem cell therapy in a mouse model of type II diabetes mellitus via the PI3K/AKT signaling pathway. Stem Cell Res Ther 2022;13:164. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 240. Qiao X, Tang J, Dou L, et al. Dental pulp stem cell-derived exosomes regulate anti-inflammatory and osteogenesis in periodontal ligament stem cells and promote the repair of experimental periodontitis in rats. Int J Nanomedicine 2023;18:4683–4703. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 241. Zhou H, Yi Z, Le D, et al. Intravenous administration of human chorionic membrane mesenchymal stem cells promotes functional recovery in a rat traumatic brain injury model. Neuroreport 2024;35:81–89. [ DOI ] [ PubMed ] [ Google Scholar ]
- 242. Du J, Zhen G, Chen H, et al. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials 2018;181:347–359. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 243. Zhang H, Li Z-L, Yang F, et al. Radial shockwave treatment promotes human mesenchymal stem cell self-renewal and enhances cartilage healing. Stem Cell Res Ther 2018;9:1–13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 244. Patten DA, Ouellet M, Allan DS, et al. Mitochondrial adaptation in human mesenchymal stem cells following ionizing radiation. FASEB J 2019;33:9263. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 245. Ginani F, Soares DM, Barreto MPEV, et al. Effect of low-level laser therapy on mesenchymal stem cell proliferation: a systematic review. Lasers Med Sci 2015;30:2189–2194. [ DOI ] [ PubMed ] [ Google Scholar ]
- 246. Chang K-A, Kim JW, Kim JA, et al. Biphasic electrical currents stimulation promotes both proliferation and differentiation of fetal neural stem cells. PLoS One 2011;6:e18738. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 247. Di L, Shi Y-N, Yan Y-M, et al. Nonpeptide small molecules from the insect Aspongopus chinensis and their neural stem cell proliferation stimulating properties. RSC Adv 2015;5:70985–70991. [ Google Scholar ]
- 248. Maul TM, Chew DW, Nieponice A, et al. Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol 2011;10:939–953. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 249. Cmielova J, Havelek R, Soukup T, et al. Gamma radiation induces senescence in human adult mesenchymal stem cells from bone marrow and periodontal ligaments. Int J Radiat Biol 2012;88:393–404. [ DOI ] [ PubMed ] [ Google Scholar ]
- 250. Wani S, Dar T, Koli S, et al. Stem cell technology in medical biotechnology. Fundamentals and Advances in Medical Biotechnology. Springer; 2022:233–267. [ Google Scholar ]
- 251. Tan D, Huang Z, Zhao Z, et al. Single‑cell sequencing, genetics, and epigenetics reveal mesenchymal stem cell senescence in osteoarthritis. Int J Mol Med 2024;53:1–17. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 252. Wang J, Zheng Y, Zhao M. Exosome-based cancer therapy: implication for targeting cancer stem cells. Front Pharmacol 2017;7:533. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 253. Zhao T, Sun F, Liu J, et al. Emerging role of mesenchymal stem cell-derived exosomes in regenerative medicine. Curr Stem Cell Res Ther 2019;14:482–494. [ DOI ] [ PubMed ] [ Google Scholar ]
- 254. Shao H, Im H, Castro CM, et al. New technologies for analysis of extracellular vesicles. Chem Rev 2018;118:1917–1950. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 255. Muthu S, Bapat A, Jain R, et al. Exosomal therapy—a new frontier in regenerative medicine. Stem Cell Investig 2021;8:7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 256. Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Comm Signal 2022;20:1–13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 257. Fayazi N, Sheykhhasan M, Soleimani Asl S, et al. Stem cell-derived exosomes: a new strategy of neurodegenerative disease treatment. Mol Neurobiol 2021;58:3494–3514. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 258. Harrell CR, Jovicic N, Djonov V, et al. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 2019;8:1605. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 259. Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Nat RevBioengineer 2023:608–609. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 260. Zhang X, Liu L. Applications of single cell RNA sequencing to research of stem cells. World J Stem Cells 2019;11:722. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 261. Yao F, Zhan Y, Li C, et al. Single-cell rna sequencing reveals the role of phosphorylation-related genes in hepatocellular carcinoma stem cells. Front Cell Dev Biol 2022;9:734287. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 262. Mollashahi B, Latifi-Navid H, Owliaee I, et al. Research and therapeutic approaches in stem cell genome editing by CRISPR toolkit. Molecules 2023;28:1982. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 263. Hussen BM, Rasul MF, Abdullah SR, et al. Targeting miRNA by CRISPR/Cas in cancer: advantages and challenges. Mil Med Res 2023;10:32. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 264. Kim G, Jeon JH, Park K, et al. High throughput screening of mesenchymal stem cell lines using deep learning. Sci Rep 2022;12:17507. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 265. Brooks IR, Garrone CM, Kerins C, et al. Functional genomics and the future of iPSCs in disease modeling. Stem Cell Reports 2022;17:1033–1047. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 266. Kantor A, McClements ME, MacLaren RE. CRISPR-Cas9 DNA base-editing and prime-editing. Int J Mol Sci 2020;21:6240. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 267. Fiumara M, Ferrari S, Omer-Javed A, et al. Genotoxic effects of base and prime editing in human hematopoietic stem cells. Nat Biotechnol 2023;42:877–891. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 268. Hamazaki T, El Rouby N, Fredette NC, et al. Concise review: induced pluripotent stem cell research in the era of precision medicine. Stem Cells 2017;35:545–550. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 269. Chandrasekaran AP, Song M, Ramakrishna S. Genome editing: a robust technology for human stem cells. Cell Mol Life Sci 2017;74:3335–3346. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 270. Sankar A, YS RK, Singh A, et al. Next-generation therapeutics for rare genetic disorders. Mutagenesis 2024;39:157–171; geae002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 271. Liu M, Yang F, Xu Y. Global trends of stem cell precision medicine research (2018–2022): a bibliometric analysis. Front Surg 2022;9:888956. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 272. Patsalias A, Kozovska Z. Personalized medicine: stem cells in colorectal cancer treatment. Biomed Pharmacother 2021;141:111821. [ DOI ] [ PubMed ] [ Google Scholar ]
- 273. Basiri A, Mansouri F, Azari A, et al. Stem cell therapy potency in personalizing severe COVID-19 treatment. Stem Cell Rev Rep 2021;17:193–213. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 274. Li H, Yang Y, Hong W, et al. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther 2020;5:1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 275. Li MD, Atkins H, Bubela T. The global landscape of stem cell clinical trials. Regen Med 2014;9:27–39. [ DOI ] [ PubMed ] [ Google Scholar ]
- 276. Trounson A, Thakar RG, Lomax G, et al. Clinical trials for stem cell therapies. BMC Med 2011;9:1–7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 277. Hernández R, Jiménez-Luna C, Perales-Adán J, et al. Differentiation of human mesenchymal stem cells towards neuronal lineage: clinical trials in nervous system disorders. Biomol Ther 2020;28:34. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 278. Kim HJ, Seo SW, Chang JW, et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase 1 clinical trial. Alzheimer’s Dement 2015;1:95–102. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 279. Kim HJ, Cho KR, Jang H, et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase I clinical trial. Alzheimer’s Res Ther 2021;13:1–11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 280. Liu Z, Cheung H-H. Stem cell-based therapies for Parkinson disease. Int J Mol Sci 2020;21:8060. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 281. Oh SK, Choi KH, Yoo JY, et al. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery 2016;78:436–447. [ DOI ] [ PubMed ] [ Google Scholar ]
- 282. Vaquero J, Zurita M, Rico MA, et al. An approach to personalized cell therapy in chronic complete paraplegia: the Puerta de Hierro phase I/II clinical trial. Cytotherapy 2016;18:1025–1036. [ DOI ] [ PubMed ] [ Google Scholar ]
- 283. Bartolucci J, Verdugo FJ, González PL, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circul Res 2017;121:1192–1204. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 284. Noiseux N, Mansour S, Weisel R, et al. The IMPACT-CABG trial: a multicenter, randomized clinical trial of CD133+ stem cell therapy during coronary artery bypass grafting for ischemic cardiomyopathy. J Thorac Cardiovasc Surg 2016;152:1582–8.e2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 285. Brown JR, Chan DK, Shank JJ, et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight 2020;5:e133247. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 286. Steenbruggen TG, Steggink LC, Seynaeve CM, et al. High-dose chemotherapy with hematopoietic stem cell transplant in patients with high-risk breast cancer and 4 or more involved axillary lymph nodes: 20-year follow-up of a phase 3 randomized clinical trial. JAMA Oncol 2020;6:528–534. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 287. Park Y-B, Ha C-W, Lee C-H, et al. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med 2017;6:613–621. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 288. Namiot ED, Niemi JVL, Chubarev VN, et al. Stem cells in clinical trials on neurological disorders: trends in stem cells origins, indications, and status of the clinical trials. Int J Mol Sci 2022;23:11453. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 289. Bolli R, Tang X-L. Clinical trials of cell therapy for heart failure: recent results warrant continued research. Curr Opin Cardiol 2022;37:193–200. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 290. Chivu-Economescu M, Rubach M. Hematopoietic stem cells therapies. Curr Stem Cell Res Ther 2017;12:124–133. [ DOI ] [ PubMed ] [ Google Scholar ]
- 291. Landeros N, Castillo I, Pérez-Castro R. Preclinical and clinical trials of new treatment strategies targeting cancer stem cells in subtypes of breast cancer. Cells 2023;12:720. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 292. Lee S, Chae D-S, Song B-W, et al. ADSC-based cell therapies for musculoskeletal disorders: a review of recent clinical trials. Int J Mol Sci 2021;22:10586. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 293. De Klerk E, Hebrok M. Stem cell-based clinical trials for diabetes mellitus. Front Endocrinol 2021;12:631463. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 294. Dragu DL, Necula LG, Bleotu C, et al. Therapies targeting cancer stem cells: current trends and future challenges. World J Stem Cells 2015;7:1185. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 295. Abusalah MAH, Chopra H, Sharma A, et al. Nanovaccines: a game changing approach in the fight against infectious diseases. Biomed Pharmacother 2023;167:115597. [ DOI ] [ PubMed ] [ Google Scholar ]
- 296. Apostolou E, Blau H, Chien K, et al. Progress and challenges in stem cell biology. Nat Cell Biol 2023;25:203–206. [ DOI ] [ PubMed ] [ Google Scholar ]
- 297. Choudhary OP, Saini J, Challana A, et al. ChatGPT for veterinary anatomy education: an overview of the prospects and drawbacks. Int J Morphol 2023;41:1198–1202. [ Google Scholar ]
- 298. Bongso A, Fong CY, Gauthaman K. Taking stem cells to the clinic: major challenges. J Cell Biochem 2008;105:1352–1360. [ DOI ] [ PubMed ] [ Google Scholar ]
- 299. Lukomska B, Stanaszek L, Zuba-Surma E, et al. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int 2019;2019:9628536. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 300. Chopra H, Choudhary OP. mRNA vaccines as an armor to combat the infectious diseases. Travel Med Infect Dis 2023;52:102550. [ DOI ] [ PubMed ] [ Google Scholar ]
- 301. Ghosh D, Mehta N, Patil A, et al. Ethical issues in biomedical use of human embryonic stem cells (hESCs). J Reproduct Health Med 2016;2:S37–S47. [ Google Scholar ]
- 302. King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther 2014;5:1–6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 303. Harris AR, Walker MJ, Gilbert F. Ethical and regulatory issues of stem cell-derived 3-dimensional organoid and tissue therapy for personalised regenerative medicine. BMC Med 2022;20:1–11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 304. Assen LS, Jongsma KR, Isasi R, et al. Recognizing the ethical implications of stem cell research: a call for broadening the scope. Stem Cell Reports 2021;16:1656–1661. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
- View on publisher site
- PDF (1.2 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Stem cells: past, present, and future
Affiliations.
- 1 Department of Experimental Surgery and Biomaterials Research, Wroclaw Medical University, Bujwida 44, Wrocław, 50-345, Poland. [email protected].
- 2 Department of Conservative Dentistry and Pedodontics, Krakowska 26, Wrocław, 50-425, Poland.
- 3 Department of Experimental Surgery and Biomaterials Research, Wroclaw Medical University, Bujwida 44, Wrocław, 50-345, Poland.
- PMID: 30808416
- PMCID: PMC6390367
- DOI: 10.1186/s13287-019-1165-5
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation. Quality control and teratoma formation assays are important procedures in assessing the properties of the stem cells tested. Derivation methods and the utilization of culturing media are crucial to set proper environmental conditions for controlled differentiation. Among many types of stem tissue applications, the use of graphene scaffolds and the potential of extracellular vesicle-based therapies require attention due to their versatility. The review is summarized by challenges that stem cell therapy must overcome to be accepted worldwide. A wide variety of possibilities makes this cutting edge therapy a turning point in modern medicine, providing hope for untreatable diseases.
Keywords: Differentiation; Growth media; Induced pluripotent stem cell (iPSC); Pluripotency; Stem cell derivation; Stem cells; Teratoma formation assay; Tissue banks; Tissue transplantation.
Publication types
- Research Support, Non-U.S. Gov't
- Cell Differentiation / genetics*
- Cell- and Tissue-Based Therapy / trends*
- Graphite / chemistry
- Graphite / therapeutic use
- Induced Pluripotent Stem Cells / transplantation*
- Stem Cell Transplantation / classification
- Stem Cells / classification
- Stem Cells / cytology*
- Tissue Scaffolds / chemistry
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS FEATURE
- 20 December 2024
Stem cells head to the clinic: treatments for cancer, diabetes and Parkinson’s disease could soon be here
- Alison Abbott 0
Alison Abbott is a writer based in Munich, Germany.
You can also search for this author in PubMed Google Scholar
A team at Skåne University Hospital in Lund, Sweden, prepares a needle to transplant cells into a person’s brain to treat Parkinson’s. Credit: Åsa Sjöström for Nature
Andrew Cassy had spent his working life in a telecommunications research department until a diagnosis of Parkinson’s disease in 2010 pushed him into early retirement. Curious about his illness, which he came to think of as an engineering problem, he decided to volunteer for clinical trials.
“I had time, something of value that I could give to the process of understanding the disease and finding good treatments,” he says.
In 2024, he was accepted into a radical trial. That October, surgeons in Lund, Sweden, placed neurons that were derived from human embryonic stem (ES) cells into his brain. The hope is that they will eventually replace some of his damaged tissue.

Stem cells reverse woman’s diabetes — a world first
The study is one of more than 100 clinical trials exploring the potential of stem cells to replace or supplement tissues in debilitating or life-threatening diseases, including cancer, diabetes , epilepsy, heart failure and some eye diseases . It’s a different approach from the unapproved therapies peddled by many shady clinics, which use types of stem cell that do not turn into new tissue.
All the trials are small and focus mainly on safety. And there are still substantial challenges, including defining which cells will be most fit for which purposes and working out how to bypass the need for immunosuppressant drugs that stop the body from rejecting the cells but increase the risk of infections.
Still, the flurry of clinical studies marks a turning point for stem-cell therapies. Following decades of intense research that has at times triggered ethical and political controversy , the safety and potential of stem cells for tissue regeneration is now being widely tested. “The rate of progress has been remarkable,” says stem-cell specialist Martin Pera at the Jackson Laboratory in Bar Harbor, Maine. “It’s just 26 years since we first learnt to culture human stem cells in flasks.”
Researchers expect some stem-cell therapies to enter the clinic soon. Treatments for some conditions, they say, could become part of general medicine in five to ten years.
Finding a source
Cassy’s symptoms began with a small, persistent tremor in his fingers when he was just 44. The characteristic motor symptoms of Parkinson’s are driven by the degeneration of dopamine-producing neurons called A9 cells in the brain’s substantia nigra. Drugs that replace the missing dopamine are effective, but have side effects including uncontrolled movements and impulsive behaviours. And as the disease progresses, the drugs’ efficacy wanes and the side effects worsen.
The idea of replacing the degenerated dopaminergic cells has a long history. During development, pluripotent ES cells, which have the potential to become many cell types, turn into the specialized cells of the brain, heart, lungs and so on. Theoretically, transplanted stem cells could repair any damaged tissue.
Parkinson’s lent itself to testing that theory. The first transplant of such cells took place in Sweden in 1987 using neurons from the developing brains of fetuses from terminated pregnancies, the only source of immature, or progenitor, neural cells at the time. Since then, more than 400 people with Parkinson’s have received such a transplant — with mixed results . Many people saw no benefit at all, or had debilitating side effects. But others improved so much that they no longer needed to take dopaminergic drugs.
Brain MRI scans of a trial participant are used to plan where the needle will deliver the cells. Credit: Åsa Sjöström for Nature
“Overall, the studies showed us that the approach can work, sometimes transformatively,” says neurologist Roger Barker at the University of Cambridge, UK. “But we needed a more-reliable source material.”
Fetal brain tissue cannot be standardized and might also be contaminated with progenitors that are destined to mature into the wrong sort of cells. On top of this, some people have ethical or religious objections to the use of this material . And in any case, notes Barker, it has often been hard to find enough material to go ahead with an operation to transplant the cells.
Prospects for regenerative stem-cell therapy improved when it became possible to derive specialized cells from more controllable sources, particularly human ES cells and, later, induced pluripotent stem (iPS) cells , which are created by reprogramming adult cells to revert to an immature state. Today, large numbers of specialized cells can be reliably produced at a quality and purity high enough for the clinic.
Stem-cell researcher Agnete Kirkeby at the University of Copenhagen and her colleagues have surveyed the landscape of regenerative-stem-cell clinical trials worldwide and, as of December 2024, they had identified 116 trials approved or completed across a range of diseases 1 . Around half use human ES cells as the starting material. The other studies use iPS cells, either off the shelf or generated from the skin cells or blood of individual people to treat their own conditions. Twelve of the trials attempt to treat Parkinson’s disease using dopamine-producing cells derived from stem cells.
Promise for Parkinson’s
The trial that Cassy is enrolled in, which Barker co-leads, and another more-advanced trial run by BlueRock Therapeutics, a biotechnology firm based in Cambridge, Massachusetts, gave participants A9 progenitor cells derived from human ES cells. The BlueRock trial has reported preliminary results for its 12 participants. Two years in, the treatment has proved safe and shown hints of efficacy in those receiving the higher of two doses. So far, no Parkinson’s trial has reported uncontrolled movement side effects such as those seen with dopaminergic drugs and in some trials that used fetal tissue.

The race to supercharge cancer-fighting T cells
Compared with other organs, such as the heart, pancreas and kidneys, the brain has proved to be one of the most straightforward organs to treat with stem cells. One advantage is that the brain is largely protected from the body’s immune system, which seeks out and destroys foreign tissue. Participants in Parkinson’s trials receive immunosuppressants for only a year to cover the period when the blood–brain barrier is healing from surgery. Participants in trials for other organs typically receive the drugs for the rest of their lives.
And the brain is accommodating. The A9 cells usually reside in the substantia nigra and send projections out to the putamen, in the forebrain, where they release dopamine. But neurosurgeons often place the progenitor cells directly in the putamen because it’s easier to get at surgically. The brain’s ability to adapt to fetal tissue and to cells transplanted into the ‘wrong’ site is “pretty clever”, says Barker.
Just as remarkable, he says, is a study of epilepsy in which transplanted cells derived from human ES cells integrate into the correct neural circuits in the brain. In the clinical trial, run by the biotechnology company Neurona Therapeutics based in San Francisco, California, surgeons transplanted immature versions of a type of brain cell called interneurons into the brains of ten people with a form of epilepsy that could not be controlled by drugs. Before receiving this treatment, the participants’ seizures were so frequent and debilitating that they could not live independently.

A rat brain with transplanted stem-cell-derived cells is put on ice and cut into slices in preparation for analysis. These cells were tested in rats before an ongoing clinical trial in people with Parkinson’s disease was approved. Credit: Åsa Sjöström for Nature
One year after the transplant, the frequency of severe seizures in the first two participants had dropped to almost zero, an effect that has been maintained for two years. Most of the other participants have had pronounced reductions in seizure frequency. There were no significant side effects and no cognitive damage, the company reports . Last June, the US Food and Drug Administration awarded the therapy a fast-track status to expedite the process that leads to regulatory approval.
“The outcomes for patients were strikingly similar even though procedures were carried out at different sites around the country,” says Arnold Kriegstein at the University of California, San Francisco, who is a co-founder of Neurona Therapeutics. “It is very robust.”
Like the brain, the eye is well-protected from the body’s immune system. Kirkeby and her colleagues identified 29 clinical trials for ocular diseases, particularly for types of age-related macular degeneration. Other organs don’t have the same immune privilege, yet are responsible for some of the most burdensome diseases, including heart failure as well as type 1 diabetes, which is caused by the destruction of insulin-producing islet cells in the pancreas.
Beyond the brain and eyes
Progress has been slower for other conditions. But positive early results from a trial run by the drug company Vertex Pharmaceuticals based in Boston, Massachusetts, have spawned a rush of optimism for diabetes. Stem-cell biologist Douglas Melton and his colleagues developed the first functional islet cells from a human ES cell line in 2014 at Harvard University in Cambridge 2 . Now at Vertex, he is leading a trial of people with particularly serious forms of the disease, using proprietary islet cells generated by similar methods. The cells do their job wherever they are placed in the body, in this case the liver. According to the company, 9 of the 12 participants who received the full dose no longer need to inject insulin, and another two were able to reduce their dose.
“I was surprised and delighted that it worked so well,” says Melton, who moved into this field in the 1990s, when his baby son was diagnosed with type 1 diabetes. “And especially glad to see the potential it has for patients.”
The laboratory of Malin Parmar at Lund University, Sweden, developed stem-cell-derived replacement cells for an ongoing trial that is attempting to use stem-cell therapy to replace damaged tissue in people with Parkinson’s disease. Credit: Åsa Sjöström for Nature
The heart has proved particularly vexing for regenerative medicine. It’s a large and complex pump made up of different cell types, and any damage must be fixed in situ . Stem-cell scientist Christine Mummery at Leiden University in the Netherlands, was one of the first to generate beating heart-muscle cells 3 , or cardiomyocytes, from human ES cells in 2002. But, she quickly realized how challenging it would be to bring to the clinic, particularly when she saw a deeply scarred and fatty heart removed during a transplant surgery. “I thought: we won’t be able to fix that any time soon.” She changed her research direction to disease modelling. But with roughly 64 million people worldwide with heart failure, Mummery says she values the persistence of those who haven’t given up.
Enjoying our latest content? Login or create an account to continue
- Access the most recent journalism from Nature's award-winning team
- Explore the latest features & opinion covering groundbreaking research
Nature 637 , 18-20 (2025)
doi: https://doi.org/10.1038/d41586-024-04160-0
Kirkeby, A. et al. Cell Stem Cell (in the press).
Paglucia, F. W. et al. Cell 159 , 428–439 (2014).
Article PubMed Google Scholar
Mummery, C. et al. J. Anat. 200 , 233–240 (2002).
Takahashi, K. & Yamanaka, S. Cell 126 , 663–676 (2006).
Download references
Reprints and permissions
Related Articles
- Therapeutics
- Cell biology
Nucleosome fibre topology guides transcription factor binding to enhancers
Article 18 DEC 24

Spatial transcriptomic clocks reveal cell proximity effects in brain ageing
Timely TGFβ signalling inhibition induces notochord
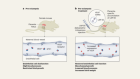
Lipid-delivery system could treat life-threatening pregnancy complication
News & Views 11 DEC 24
Next-generation snakebite therapies could reduce death toll
Outlook 21 NOV 24
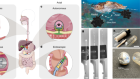
Cephalopod-inspired jetting devices for gastrointestinal drug delivery
Article 20 NOV 24
Engineered extrachromosomal oncogene amplifications promote tumorigenesis
A lipid made by tumour cells reprograms immune cells
Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumours
Article 11 DEC 24
Professor/Associate Professor/Assistant Professor/Senior Lecturer/Lecturer
The School of Science and Engineering (SSE) at The Chinese University of Hong Kong, Shenzhen (CUHK-Shenzhen) sincerely invites applications for mul...
Shenzhen, China
The Chinese University of Hong Kong, Shenzhen (CUHK Shenzhen)
Faculty Positions at SUSTech Department of Biomedical Engineering
We seek outstanding applicants for full-time tenure-track/tenured faculty positions. Positions are available for both junior and senior-level.
Shenzhen, Guangdong, China
Southern University of Science and Technology (Biomedical Engineering)
Faculty Positions in Nonhuman Primate Research, School of Life Sciences, Westlake University
SLS invites applications for multiple tenure-track/tenured faculty positions at all academic ranks.
Hangzhou, Zhejiang, China
School of Life Sciences, Westlake University
Postdoctoral Research Fellows at Suzhou Institute of Systems Medicine (ISM)
ISM, based on this program, is implementing the reserve talent strategy with postdoctoral researchers.
Suzhou, Jiangsu, China
Suzhou Institute of Systems Medicine (ISM)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
COMMENTS
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and ...
Feb 26, 2019 · In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and ...
New therapies, based on stem cell transplantation or endogenous stem cells, are emerging areas, as is drug discovery based on patient-specific pluripotent cells and cancer stem cells. What makes stem cell research so exciting is its tremendous potential to benefit human health and the opportunities for interdisciplinary research that it presents.
Stem cells’ immunomodulatory actions have undergone extensive research when contrasted with other stem cell types 65,66. Stem cells have a role in suppressing acute-phase responses by suppressing excessive activation of macrophages and T cells and initiating the secretion of inflammatory cytokines.
Dec 11, 2024 · Stem cells are cells that have the capacity to self-renew by dividing and to develop into more mature, specialised cells. Stem cells can be unipotent, multipotent, pluripotent or totipotent ...
Stem Cell Research is dedicated to publishing high-quality manuscripts focusing on the biology and applications of stem cell research. Submissions to Stem Cell Research, may cover all aspects of stem cells, including induced pluripotent stem cells, embryonic stem cells, tissue-specific stem cells, cancer stem cells, developmental studies, and genomics or translational research.
Feb 26, 2019 · In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The gen …
5 days ago · Stem-cell research is the area of research that studies the properties of stem cells and their potential use in medicine. As stem cells are the source of all tissues, understanding their ...
3 days ago · More than 100 clinical trials put stem cells for regenerative medicine to the test. ... In a particularly fast-growing area of clinical research, immune cells generated from pluripotent stem cells ...
Mar 5, 2022 · From 1971 to 2021, 40,183 research papers were published regarding stem-cell-based therapies. All of these studies were conducted around discoveries and for the goal of “Stem Cell Therapy” based on the therapeutic efficacy of stem cells . As basic stem cell research has soared over the past few years, “translation research”, a ...