Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms
- Department of Health and Human Services
- National Institutes of Health


COVID-19 Research Studies
More information, about clinical center, clinical trials and you, participate in a study, referring a patient, about clinical research.
Research participants are partners in discovery at the NIH Clinical Center, the largest research hospital in America. Clinical research is medical research involving people The Clinical Center provides hope through pioneering clinical research to improve human health. We rapidly translate scientific observations and laboratory discoveries into new ways to diagnose, treat and prevent disease. More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more , to see if clinical studies are for you.
Medical Information Disclaimer
Emailed inquires/requests.
Email sent to the National Institutes of Health Clinical Center may be forwarded to appropriate NIH or outside experts for response. We do not collect your name and e-mail address for any purpose other than to respond to your query. Nevertheless, email is not necessarily secure against interception. This statement applies to NIH Clinical Center Studies website. For additional inquiries regarding studies at the National Institutes of Health, please call the Office of Patient Recruitment at 1-800-411-1222

Find NIH Clinical Center Trials
The National Institutes of Health (NIH) Clinical Center Search the Studies site is a registry of publicly supported clinical studies conducted mostly in Bethesda, MD.

- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

You are here
Research & training.
Advances in Colorectal Cancer Research
Clinical Trials
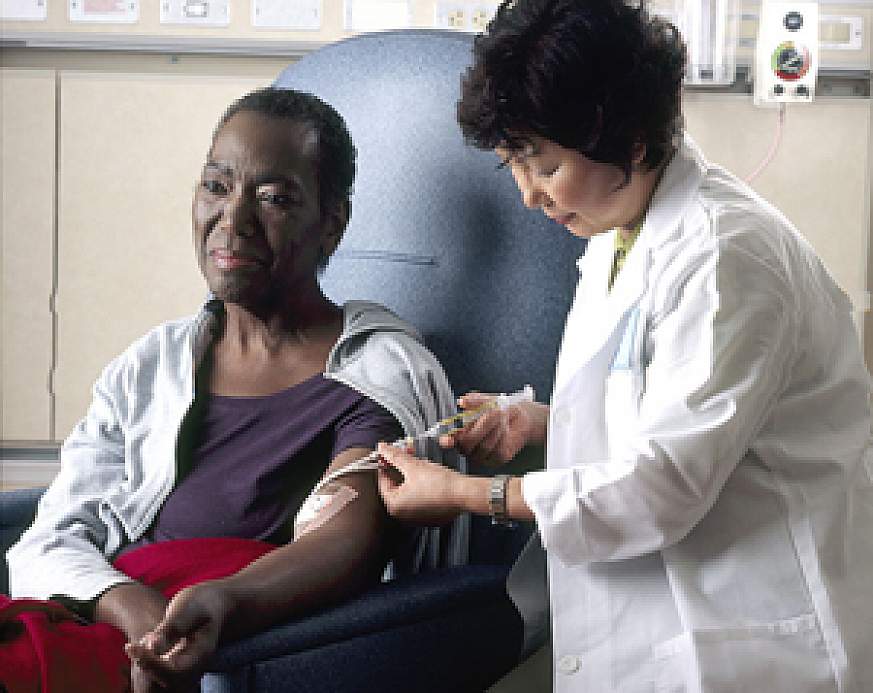
Clinical trials are research studies that involve people and test new ways to prevent, detect, diagnose, or treat diseases. Many medical procedures and treatments used today are the result of past clinical trials.
Taking part in a clinical trial has potential benefits and risks. The potential benefits of participating in a trial include the following:
- Trial participants have access to promising new procedures or treatments that are generally not available outside of a clinical trial.
- The new procedure or treatment being studied may be more effective than the current usual approach. If it is more effective, trial participants may be the first to benefit from it.
- Trial participants receive high-quality medical care from a research team that includes doctors, nurses, and other health professionals.
- The results of the trial may help other people who need medical care in the future.
- Trial participants are helping scientists learn more about cancer and other medical conditions, which will lead to more advances.
Some of the potential risks of taking part in a clinical trial are:
- The new procedure or drug may not be better than what is currently available, or it may have side effects that doctors do not expect or that are worse than the side effects of the current usual approach.
- Trial participants may be required to make more visits to the doctor than they would if they were not in a clinical trial and/or need to travel farther for those visits.
- Some of the costs of participating in a trial may not be covered by health insurance.
The decision to take part in a clinical trial is a personal one. Your health care team and your loved ones, if you wish, can assist you in deciding whether or not a clinical trial is right for you. The final decision, however, is yours alone to make.
Visit ClinicalTrials.gov to search for NIH-sponsored colorectal cancer clinical trials that are currently accepting patients.
This page last reviewed on August 20, 2015
Connect with Us
- More Social Media from NIH

IMAGES
COMMENTS
Glossary. Study record managers: refer to the Data Element Definitions if submitting registration or results information.. Search for terms
Nov 6, 2018 · NIH conducts clinical research trials for many diseases and conditions, including cancer, Alzheimer’s disease, allergy and infectious diseases, and neurological disorders. To search for other diseases and conditions, you can visit ClinicalTrials.gov.
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease.
More than 500,000 people from around the world have participated in clinical research since the hospital opened in 1953. We do not charge patients for participation and treatment in clinical studies at NIH. In certain emergency circumstances, you may qualify for help with travel and other expenses Read more, to see if clinical studies are for you.
Aug 20, 2015 · Clinical trials are research studies that involve people and test new ways to prevent, detect, diagnose, or treat diseases. Many medical procedures and treatments used today are the result of past clinical trials. Taking part in a clinical trial has potential benefits and risks. The potential benefits of participating in a trial include the ...
For the purposes of registration, a clinical trial is any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes. Clinical trials may also be referred to as interventional trials.