Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 22 August 2022

A systematic literature review to clarify the concept of vaccine hesitancy
- Daphne Bussink-Voorend ORCID: orcid.org/0000-0002-9873-1404 1 ,
- Jeannine L. A. Hautvast 1 ,
- Lisa Vandeberg ORCID: orcid.org/0000-0002-7229-2378 2 ,
- Olga Visser 1 &
- Marlies E. J. L. Hulscher ORCID: orcid.org/0000-0002-2160-4810 3
Nature Human Behaviour volume 6 , pages 1634–1648 ( 2022 ) Cite this article
22k Accesses
89 Citations
59 Altmetric
Metrics details
- Human behaviour
- Infectious diseases
- Preventive medicine
Vaccine hesitancy (VH) is considered a top-10 global health threat. The concept of VH has been described and applied inconsistently. This systematic review aims to clarify VH by analysing how it is operationalized. We searched PubMed, Embase and PsycINFO databases on 14 January 2022. We selected 422 studies containing operationalizations of VH for inclusion. One limitation is that studies of lower quality were not excluded. Our qualitative analysis reveals that VH is conceptualized as involving (1) cognitions or affect, (2) behaviour and (3) decision making. A wide variety of methods have been used to measure VH. Our findings indicate the varied and confusing use of the term VH, leading to an impracticable concept. We propose that VH should be defined as a state of indecisiveness regarding a vaccination decision.
Similar content being viewed by others

A scoping review of global COVID-19 vaccine hesitancy among pregnant persons
An effective COVID-19 vaccine hesitancy intervention focused on the relative risks of vaccination and infection

Vaccination mandates and their alternatives and complements
In 2019, vaccine hesitancy (VH) was named by the World Health Organization (WHO) as one of the top-10 threats to global health, following a five-fold global increase in measles, a disease that can be prevented by vaccination 1 , 2 . The largest increase was reported in the WHO regions covering Europe and the Americas 2 . The impact of these measles outbreaks is substantial, with rises in morbidity, mortality and costs 3 , 4 , 5 . The increasing incidence of measles and other vaccine-preventable diseases has been attributed to a failure to reach adequate immunization coverage rates 2 , 6 . In the European region, VH has been identified as the main barrier to vaccination coverage 7 , 8 . This is in contrast to other regions, such as sub-Saharan Africa, where immunization coverage rates are challenged by a combination of barriers, including access and availability 9 .
In the past decade, VH has become a key topic of research in various fields, following rises in vaccine-preventable diseases, the introduction of new vaccines, the spread of misinformation and lagging vaccination coverage 10 . Moreover, the COVID-19 pandemic has drawn further attention to the role of VH in limiting the uptake of vaccines and failure to achieve collective immunity 11 , 12 , 13 . This has led to the proliferation of scientific literature on VH in the public health, biomedical and social science research fields 10 .
In 2012, the WHO established a strategic advisory group of experts (SAGE) working group with the mandate of defining VH and suggesting how to monitor and address it. The working group proposed a broad definition, describing a VH continuum from acceptance to refusal of vaccines or as a delay in acceptance or refusal despite the availability of the vaccines. The working group described VH as “A complex behavioural phenomenon specific to vaccines, context, time, and place and influenced by factors of complacency, convenience, and confidence” 14 . This broad definition emphasizes variability by describing that VH may vary between types of vaccines and different contexts, may change over time or between different geographical locations and is influenced by various determinants.
The concept of VH has been described and applied in various ways. When definitions are broad and lack clarity, this can lead to the emergence of different concepts with overlapping domains, with various concepts being used interchangeably by some and recognized as distinct entities by others 15 . Additionally, lack of conceptual clarity can lead to inadequate operationalization and cause confusion among researchers 15 . This is problematic because when studies use similar terminology with a different meaning, their results are incomparable across subgroups, locations or contexts. A clear conceptualization is needed to develop meaningful measures allowing comparison of results 16 .
A lack of conceptual clarity is observed in the literature on VH, where VH is variously conceptualized as a psychological state and as different types of vaccination behaviour 17 , 18 . In addition, the terms ‘vaccine confidence’, ‘low uptake’ and ‘low intention to vaccinate’ are often equated with VH 19 , 20 . Confusion among researchers is then illustrated by inconsistencies in the applied definitions 21 , 22 . It has even been argued that VH is a catch-all category, aggregating many different concepts rather than being one measurable construct; and this is impeding progress in the research field 23 .
A good concept definition consists of characteristics, attributes or features that are unique to that concept and distinguish it from other closely related concepts 15 . Given the importance of VH for predicting and influencing individual vaccination decisions, it is important to explore the uses of VH and propose an optimal operationalization, distinguishing VH from other closely related concepts. Such clarification could enable a universally adopted definition and aid further research in this area.
The purpose of this systematic review was to provide an overview of how VH is operationalized in the literature in terms of conceptualizations, subpopulations and measurements. Following an assessment of the various conceptualizations, we differentiated the common themes, related concepts, research fields and vaccine types. The scope and structure of this systematic review is visualized in Fig. 1 . On the basis of an interpretation of these findings, we suggest a way forward by proposing a renewed definition for VH.
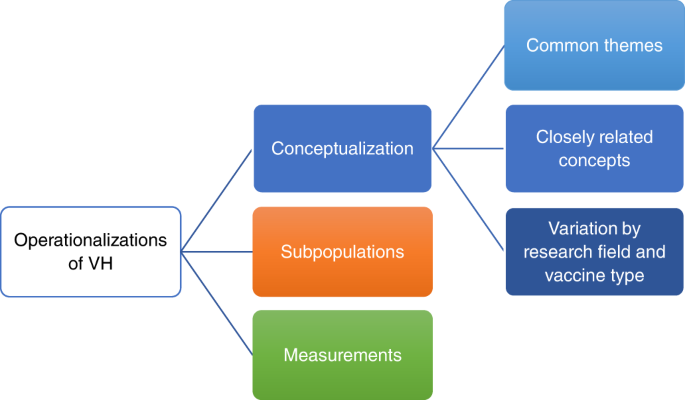
Aiming to give an overview of VH, we recognize three types of operationalizations: conceptualizations (blue), identification of subpopulations (orange) and measurements (green). Conceptualizations of VH are analysed at three levels: (1) common themes, (2) closely related concepts and (3) potential variation in conceptualization between research field and vaccine type. Each type of operationalization and its levels are discussed in separate sections.
Study selection and characteristics
The search strategy yielded 7,427 publications. After screening the titles and abstracts, 919 publications were selected for full-text screening. A total of 420 publications met the inclusion criteria. Seven additional studies were found through citation searching, two of which met the inclusion criteria, adding up to a total of 422 studies. Some studies met the criteria of more than one category, with 36 studies categorized under VH conceptualizations, 63 under VH subpopulations and 373 under VH measurements. The search process is summarized in the PRISMA flow chart (Fig. 2 ) 24 . The characteristics of included studies are described in more detail in Supplementary Table 1 .
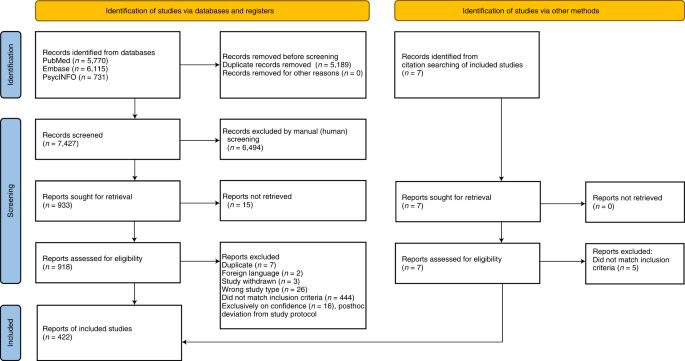
Visualization of the process involving identification of records from databases, screening of records, assessing reports for eligibility, inclusion of eligible studies and exclusion of non-eligible reports with reasons for exclusion. The number of records or reports in each step of the process is shown in brackets.
The included studies cover a wide geographical distribution. The limited majority (54%) originated in high-income countries (HIC), mainly the United States, Canada, Italy, Australia and France. A smaller group (43%) originated in low- and middle-income countries (LMICs), primarily China, India and Turkey. The remaining studies (3%) originated in a combination of HIC and LMICs. The majority (60%) were published in 2021 and 2022.
The included studies approach VH in relation to various vaccine types: 51% pertaining to COVID-19, 29% to childhood, 4% to human papillomavirus, 4% to influenza and 2% to miscellaneous vaccines. Additionally, 11% of the studies concern vaccines in general. Various research fields are represented, including public health (43%), biomedical science (30%), paediatrics (15%) and social sciences (12%). Mixed methods appraisal tool (MMAT) scores were calculated for 88% of the included studies, while the others could not be assessed due to their study types. The majority (68%) scored 3 or higher, indicating that 60% of the quality criteria were met.
Vaccine hesitancy conceptualization
From the 36 studies on VH conceptualization, we extracted and analysed 304 excerpts. Supplementary Table 2 shows the extracted text excerpts for each study. Our thematic analysis revealed that 93 excerpts describe an overall characterization of VH. The majority of these (69%) describe the nature of VH as heterogenous 14 , 21 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , complex 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 29 , 33 , 35 , 38 , 39 , 40 , 41 , 42 , 43 or varied, depending on the type of vaccine and the context 14 , 18 , 20 , 21 , 23 , 27 , 28 , 30 , 33 , 35 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 .
VH is conceptualized in 208 excerpts. The thematic analysis revealed three predominant conceptualizations in 165 (79%) excerpts: cognitions or affect, behaviour and decision making. These three conceptualizations overlap in the majority of the studies and excerpts. Illustrative excerpts of each conceptualization are presented in Table 1 . The remaining 45 (22%) excerpts represent a fragmented group of conceptualizations, without emerging themes.
Vaccine hesitancy conceptualized as cognitions or affect
From all 36 studies 14 , 17 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 98 excerpts were extracted as conceptualizing VH in terms of cognitions or affect, including questioning, emotions or beliefs regarding vaccination. For this conceptualization, we rank-ordered the most frequently used descriptions of VH, including having or expressing concerns 21 , 25 , 26 , 27 , 29 , 30 , 34 , 35 , 36 , 40 , 42 , 43 , 46 , 51 , 53 , doubts 21 , 28 , 29 , 36 , 43 or questions 21 , 26 , 47 and being reluctant 23 , 27 , 29 , 32 , 36 , 38 , 45 , 49 , 53 , 54 or unsure 14 , 21 , 27 , 29 , 34 . Many authors describe VH as pertaining to beliefs 34 , 49 , attitudes 21 , 26 , 37 , 43 , 51 or both 23 , 29 , 30 , 55 . Furthermore, vaccine-hesitant individuals are described as ambivalent to vaccination or perceiving ambiguity in vaccine-related risks 21 , 36 , 50 , 53 .
Vaccine hesitancy conceptualized as behaviour
From 35 studies 14 , 17 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 94 excerpts were extracted as conceptualizing VH as a behaviour. The majority of the excerpts describe VH in terms of various behaviours 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 29 , 31 , 32 , 34 , 35 , 37 , 38 , 39 , 40 , 41 , 44 , 45 , 51 , as illustrated by the following example: “VH refers to a ‘delay’ in acceptance or ‘refusal’ of vaccines” 14 . Other excerpts describe VH as a range or continuum between the extreme ends of accepting all vaccines and refusing all vaccines 21 , 22 , 27 , 28 , 29 , 30 , 31 , 33 , 36 , 38 , 43 . In a minority of the excerpts, VH is described as a specific type of vaccination behaviour, including vaccinating as recommended (despite reluctance, concerns or feeling unsure) 26 , 46 , 47 , 49 , refusing vaccines 28 or delaying vaccines and choosing an alternative schedule 50 . Some studies explicitly state that VH should not be described as a vaccination behaviour 17 , 18 , 36 , 40 . Within articles, there were inconsistencies in the behavioural descriptions of VH 18 , 22 , 26 , 27 , 28 , 29 , 31 , 38 , 41 .
Vaccine hesitancy conceptualized as decision making
From 19 studies 18 , 21 , 23 , 26 , 27 , 30 , 31 , 32 , 36 , 37 , 38 , 40 , 42 , 44 , 45 , 50 , 52 , 53 , 30 excerpts were extracted as conceptualizing VH in terms of vaccine decision-making. Some authors adopt the term VH when describing individuals who are undecided, indecisive or under consideration, and not yet having made a final vaccine decision 21 , 23 , 26 , 31 , 32 , 45 , 50 . Vaccine-hesitant individuals are described as being in various states of indecision 23 , 31 , 32 , 37 or as seeking more information to make ‘the right decision’ about vaccination 21 , 53 . Moreover, some authors describe VH as an approach to 38 or a transient stage in the process of vaccine decision-making itself 21 , 23 , 37 .
Vaccine hesitancy and related concepts
VH is often described in relation to other concepts. We extracted 142 excerpts from 31 studies describing closely related concepts 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 29 , 30 , 32 , 33 , 34 , 35 , 36 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 50 , 51 , 52 , 53 . The three most common concepts are confidence or trust, complacency and convenience. Together, these are referred to as ‘the 3 Cs’ 14 and described in 69 of 142 (49%) excerpts. Most often, the 3 Cs are described as having a causal relationship with VH and as representing determinants 14 , 18 , 20 , 29 , 33 , 35 , 38 , 41 , 48 , 56 .
From 25 studies, 46 excerpts were extracted as describing confidence 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 29 , 30 , 33 , 34 , 35 , 36 , 38 , 39 , 41 , 42 , 43 , 44 , 46 , 47 , 48 , 52 . ‘Confidence’ is defined as the trust that people have in the immunizations, the healthcare system itself, and the process leading to decisions on licensing or recommended schedules 14 , 27 , 35 . Few studies describe the (lack of) trust or confidence as a component of VH 23 , 34 , 52 .
From 22 studies 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 29 , 30 , 33 , 35 , 38 , 39 , 40 , 41 , 43 , 44 , 47 , 48 , 50 , 52 , 41 excerpts were extracted on the theme of complacency. ‘Complacency’ is the individual evaluation of the risks and benefits of vaccines and of the need to vaccinate 14 , 18 , 20 , 35 . The concept of complacency in relation to VH is described as the tendency to perceive the risks of vaccination as unknown or disproportionally high and the risks of the vaccine-preventable disease as low 44 , 50 . Vaccine-hesitant individuals are more committed to assessing vaccine risks and seeking ways to minimize them 23 , 40 , 47 , 50 .
From 15 studies 14 , 18 , 20 , 21 , 22 , 25 , 29 , 33 , 35 , 38 , 39 , 41 , 42 , 43 , 48 , 27 excerpts were extracted as describing the theme of convenience. ‘Convenience’ concerns not only physical availability and geographical accessibility of vaccines, but also the user-friendliness of and ability to understand immunization services 14 , 18 , 35 , 42 . In our analysis, we found that many authors refer to convenience by describing VH as the delaying or refusal of vaccines ‘despite availability’ 14 , 18 , 21 , 22 , 23 , 25 , 26 , 29 , 33 , 35 , 38 , 39 , 41 . This description acknowledges that availability of vaccines is related to vaccine uptake, while VH itself is not influenced by availability issues. However, one study adopts inconvenience and difficulty to access vaccines as dimensions of VH 42 .
Variations between research fields and vaccine types
We identified the respective research field and vaccine type of each study in the qualitative analysis to explore related differences in descriptions of VH. We identified 19 public health studies 18 , 21 , 23 , 25 , 26 , 27 , 28 , 29 , 32 , 33 , 36 , 37 , 38 , 41 , 45 , 47 , 50 , 51 , 53 , 6 paediatric studies 14 , 31 , 34 , 35 , 39 , 48 , 8 social science studies 17 , 20 , 22 , 42 , 44 , 46 , 49 , 52 and 3 biomedical studies 30 , 40 , 43 . The primary difference observed was that conceptualizations of VH in terms of decision making emerged predominantly in the public health 18 , 21 , 23 , 32 , 38 , 50 , 54 and social science fields 42 , 44 , 52 . In studies conceptualizing VH in terms of cognitions or affect, the terms ‘beliefs’ and ‘concerns’ were used in all research fields, while ‘reluctance’, ‘doubts’ and ‘questions’ were used almost exclusively in the public health field. The conceptualization of VH as a behaviour occurred in all research fields.
VH was discussed in relation to vaccination in general 14 , 17 , 18 , 22 , 23 , 27 , 28 , 29 , 32 , 33 , 35 , 36 , 38 , 41 , 42 , 43 , 46 , 48 , 49 or specifically with regard to childhood vaccines 21 , 25 , 26 , 30 , 31 , 34 , 37 , 39 , 40 , 47 , 50 , 51 , 53 , in 19 and 13 of the studies, respectively. The remaining 4 studies discussed VH in relation to COVID-19 44 , 45 , 52 and influenza 20 . Our analysis compared the studies on general vaccination and childhood vaccines but found no major differences in their respective conceptualizations.
Vaccine hesitancy subpopulations
Of the 422 included studies, 63 identified various VH subpopulations. We extracted text excerpts describing the classifications of these subpopulations and the authors’ rationales for the distinctions. The analysis identified themes aligned with the three VH conceptualization categories. Fourteen studies grouped VH subpopulations on the basis of criteria from the conceptualization as cognitions or affect 21 , 23 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 and 3 studies grouped VH on the basis of the conceptualization of decision making 69 , 70 , 71 . VH subpopulations grouped solely on the basis of criteria from the behaviour conceptualization were not found. However, 19 studies grouped hesitant individuals on the basis of criteria from the conceptualizations of both cognitions or affect, and behaviour 26 , 47 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 . The remaining 27 studies did not identify subpopulations in terms of the three conceptualizations. Twelve studies identified subpopulations on the basis of degree of VH 51 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 . Although degree of VH does not directly contribute to understanding of the VH concept, the instruments used to quantify it and determine cut-off values for the subpopulations contain valuable information about the operationalizations. These instruments are discussed in the following section. In addition, a group of 10 studies distinguished a VH subpopulation by asking about willingness to be vaccinated but used different criteria to do so 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 . This method was mainly found in studies on COVID-19 vaccination, published in 2021. This demonstrates the emergence of a conceptual VH category that was not identified from the conceptual studies. The final 5 studies grouped subpopulations according to miscellaneous criteria 45 , 49 , 110 , 111 , 112 . An overview is provided Supplementary Table 3 .
Measurements of vaccine hesitancy
Of the 422 studies included, 373 report a measurement of VH in individuals. An overview is provided in Supplementary Table 4 , grouping the studies according to the instruments used. The most common, albeit highly heterogenous, method used in 210 (56%) studies is a brief VH assessment comprising 1–3 questions 64 , 65 , 66 , 68 , 71 , 74 , 75 , 84 , 85 , 88 , 90 , 96 , 97 , 98 , 100 , 102 , 103 , 105 , 106 , 107 , 108 , 109 , 111 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 , 291 , 292 , 293 , 294 , 295 , 296 , 297 , 298 . The questions, as well as the criteria or cut-off points used to define hesitancy, vary widely between the studies. The majority of questions used in this method cover operationalizations of VH that did not emerge from our conceptual analysis, including intention and willingness. A group of 124 studies assess VH by asking about vaccination intention. For example, one measurement asks “What would you do if a COVID-19 vaccine were available?”. Respondents answering either “I would eventually get a vaccine, but wait a while first”, “I would not get a vaccine” or “I’m not sure” are all classified as hesitant 169 . A group of 35 studies assess VH by asking about willingness, exemplified by the question: “Are you willing to receive the COVID-19 vaccination?”. Respondents answering “yes, but I choose to delay timing of injection” are considered hesitant 100 . Furthermore, 23 studies assess VH by an explicit verbatim assessment of experienced hesitancy levels. This is exemplified by the question: “Overall, how hesitant about childhood vaccines would you consider yourself to be?”. Respondents answering “not too hesitant”, “not sure”, “somewhat hesitant” or “very hesitant” are considered hesitant 136 . Finally, a minority of 14 studies assess VH with questions covering conceptualizations that did emerge from our conceptual analysis; for example, by asking about previous vaccination behaviour: “Have you ever hesitated, delayed, or refused getting a vaccination for your child or yourself due to reasons other than allergies and sickness?”. Respondents answering “yes” to this question are considered hesitant 122 . The remaining 14 studies use miscellaneous questions to assess VH. Notably, the intention and willingness measures to assess VH are found mainly in studies published in 2021 on COVID-19 vaccination, while the other methods have been used throughout the covered period and in the context of different vaccines.
The second most common method, applied by 132 (35%) studies, is the use of a validated instrument. The most common instrument, used in 70 studies, is the parent attitudes about childhood vaccines (PACV) survey, introduced by Opel et al. 34 . The PACV consists of 15 questions about immunization behaviour, beliefs about vaccine safety and efficacy, attitudes toward vaccine mandates and exemptions, and trust 299 , thereby operationalizing VH as both cognitions or affect, and behaviour. Trust (or confidence) is also included in this instrument. In our conceptual analysis, confidence emerged as a distinct concept, albeit closely related to VH. Clear cut-off points for hesitancy were formulated and applied in the vast majority of the studies using this instrument (shown in Supplementary Table 4 ). The PACV is variously used in its original form 34 , 91 , 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 , 308 , 309 , 310 , 311 , 312 , 313 , 314 , 315 , 316 , 317 , 318 , 319 , 320 , 321 , 322 , 323 , 324 , 325 , 326 , 327 , 328 , 329 , 330 , 331 , 332 , 333 , 334 , 335 , 336 , 337 , 338 , or in adapted 339 , 340 , 341 , 342 , 343 , 344 , 345 , 346 , 347 , 348 , 349 , 350 , 351 , 352 , 353 , 354 , 355 or shorter versions 51 , 62 , 89 , 93 , 95 , 356 , 357 , 358 , 359 , 360 , 361 .
Other studies use a variety of validated and broadly used instruments. The SAGE instrument is applied in 13 of the studies 41 , 362 , 363 , 364 , 365 , 366 , 367 , 368 , 369 , 370 , 371 , 372 , 373 , with questions reflecting the different conceptualizations (cognitions or affect, behaviour and decision making) and related concepts including convenience, complacency and confidence 41 . The vaccine hesitancy scale (VHS), used in 39 studies 83 , 99 , 374 , 375 , 376 , 377 , 378 , 379 , 380 , 381 , 382 , 383 , 384 , 385 , 386 , 387 , 388 , 389 , 390 , 391 , 392 , 393 , 394 , 395 , 396 , 397 , 398 , 399 , 400 , 401 , 402 , 403 , 404 , 405 , 406 , 407 , 408 , 409 , 410 , was derived from a subscale of the SAGE instrument, narrowed to conceptualize VH as cognitions or affect and include the related concept of confidence 69 . The studies using the SAGE instrument and VHS use varying outcomes or cut-off values (or no outcomes or cut-off values at all) to define hesitancy (shown in Supplementary Table 4 ). The Oxford COVID-19 vaccine hesitancy scale was recently designed exclusively for the assessment of VH for COVID-19 vaccination and subsequently applied in 5 studies 44 , 411 , 412 , 413 , 414 . Other instruments described in the context of VH but intended to assess other concepts include the 5C scale 22 of psychological antecedents of vaccine behaviour, the vaccine acceptance scale (which covers the domains cognitions and affects, confidence and legitimacy of government vaccine mandates 46 ) and the multidimensional vaccine hesitancy scale covering perceptions regarding vaccines in general 42 . Instruments assessing confidence have also been applied to assess hesitancy 415 .
The remaining 31 (8%) studies use a variety of unique, self-developed methods to measure hesitancy. These are classified as ‘miscellaneous’ 25 , 50 , 52 , 69 , 73 , 92 , 94 , 416 , 417 , 418 , 419 , 420 , 421 , 422 , 423 , 424 , 425 , 426 , 427 , 428 , 429 , 430 , 431 , 432 , 433 , 434 , 435 , 436 , 437 , 438 , 439 . Examples include measurement of VH based on vaccination rates from medical records 418 and statistical procedures used to group participants according to their patterned responses to a questionnaire 92 , 439 .
Our systematic review reveals that VH is conceptualized in the literature as involving cognitions or affect, behaviour and decision making, representing three distinct but interacting entities. Closely related concepts include confidence or trust, perceptions of the need to vaccinate and of risk (complacency), and convenience. VH subpopulations are grouped according to a variety of criteria, with the majority originating in the three identified conceptualizations. Studies measuring VH have used a wide variety of instruments. The most commonly applied instruments include a brief assessment comprising 1–3 variable questions and the PACV for childhood vaccines. The instruments operationalize hesitancy using one or more of the three identified conceptualizations, but also introduce novel conceptualizations including intention and willingness. When synergizing the findings on different VH operationalizations, we found psychological and behavioural operationalizations, with the psychological operationalizations being cognitions or affect, and decision making.
Our findings illustrate the challenge of operationalizing VH, with studies adopting different conceptualizations, subpopulations and measurements. Dubé et al. acknowledged this challenge of operationalizing the VH concept due to its heterogeneity and the diversity in attitudes and behaviours 29 . Furthermore, our findings align with a recent study demonstrating the many interpretations of VH used across Europe 440 . These inconsistencies in terminology are even evidenced in the Merriam-Webster dictionary, where ‘hesitancy’ is defined as a quality or state of being that involves indecision or reluctance 441 , aligning with VH conceptualized as decision making and cognitions or affect, while ‘vaccine hesitancy’ is defined as the reluctance or refusal to vaccinate 442 , thereby also including a conceptualization of behaviour.
In the introduction, we describe interchangeable use of various terms with VH 19 , 20 . In our review, we also found numerous examples, including ‘confidence’ 443 , ‘low intention’ 444 and ‘unwillingness’ 270 . We identify these concepts as related but not synonymous to VH. For instance, some authors note that confidence or trust are used interchangeably in relation to VH 19 , 22 , suggesting equivalent meanings. Others describe an inverse relationship, meaning that lower levels of confidence are associated with higher levels of VH 19 , 33 , 54 , 56 , 445 . In line with this, VH is described as originating from a lack of confidence 446 and as a possible indicator of declining confidence 56 .
Additionally, in our analysis of subgroups and measurements, we found that VH is frequently operationalized in terms of willingness and intention, which we did not find in our conceptual analysis of VH. Willingness and intention to vaccinate, similar to the ‘vaccine confidence’ concept, are inversely related concepts that are unequivocally linked to VH but are and should not be treated as synonymous. Using these terms interchangeably is not only inappropriate but also contributes to confusion and unclarity of the VH concept. This clarity is needed because unclear concepts give rise to differences in our understanding of its determinants, correlates and consequences, hindering efforts to study and address VH 15 , 23 , 440 . Furthermore, at an operational level, there may be a mismatch between a concept and its measures 15 . This is demonstrated in our review by the highly variable methods we found to measure VH, leading to incomparable results. Particularly during 2021, there has been a plethora of studies reporting VH measurements that, due to divergent definitions and methods, have been of questionable value. As a way forward, we base our reasoning for a renewed definition of VH on the three main identified conceptual categories—behaviour, cognitions or affect, and decision making—as these have proven most promising by their repeated representation in conceptual, subgroup and measurement studies
We argue that conceptualizing VH as vaccination behaviour is untenable, as mere behaviour is insufficiently discriminating between hesitant and non-hesitant individuals. For instance, people may accept vaccines with or without hesitation or reject vaccines with or without hesitation. As concepts are ideally defined by a unique set of features that distinguishes them from other closely related concepts 15 , vaccination behaviour alone is not sufficient to define VH. Furthermore, vaccination behaviour is generally used as the indicator of (non-)acceptance of vaccination. Thus, to use this also to define another concept would create confusion. Authors have commented on the blurred distinction between VH and refusal of vaccines 25 , 39 and criticized behavioural operationalization for its failure to capture VH 17 , 18 , 23 , 25 , 40 . Although we agree that certain types of vaccination behaviour may be manifestations of VH, we argue that including behaviour in the definition and operationalization of VH is neither necessary nor sufficient.
Our analysis shows that VH is furthermore defined by two closely linked conceptualizations that we identify as psychological—cognitions or affect, and decision making. Larson et al. exemplify this stance, arguing that VH is by nature a state of indecision and reluctance 32 . We propose to reject types of vaccination behaviour as a viable conceptualization of VH; this logically results in the proposition that VH should be considered a psychological construct. This is in line with authors who have argued that VH is a psychological state rather than a behaviour 18 , 22 , 26 , 32 , 40 , inspiring our current investigation of what exactly this vaccine-hesitant state entails. In the conceptualization cognitions or affect, VH is mainly described as ‘doubts’, ‘concerns’ and ‘reluctance’ regarding vaccination. Following our analysis, we interpreted these descriptions as different ways of how VH may be affected, experienced or expressed at an individual level, representing a layer surrounding the central element of VH. We therefore interpret cognitions and affect to go hand-in-hand, but not to be at the core of hesitancy. Moreover, we conclude that cognitions or affect are insufficiently distinctive to define VH.
This interpretation does not mean that the identified cognitions or affect are irrelevant to VH. On the contrary, they may prove crucial in shaping VH. However, to arrive at a clear definition of VH, cognitions and affects should be treated as clearly defined entities as well. Only by unravelling and distinguishing them can the exact nature of their relationship with VH be clarified in further research.
In the conceptualization decision making, VH was described as being ‘undecided’, ‘indecisive’, ‘in consideration’ or ‘not yet making a vaccine decision’. All these descriptions include an element of indecision, and this provides a unique and distinctive feature for VH. Additionally, we found that this conceptualization is predominantly discussed in studies in the public health field. This is rather logical, as one would expect this field of research to take a more pragmatic approach, examining the presence of VH at a stage where people have been offered a vaccine or to anticipate public sentiments around willingness to accept a vaccine when it is offered. This probably triggers a decision-making process where VH can emerge and manifest. On the basis of these findings, we argue that VH is a psychological state of being undecided, indecisive or not yet making a decision regarding vaccination.
The study selection was conducted independently by different members of our research team. However, one possible limitation is that we did not attempt to exclude studies of lower quality, as we wanted to maintain a robust selection of studies to enable a broad overview of the relevant literature. Our MMAT assessment, however, indicates that the majority of the studies are of medium quality. A second limitation is that a considerable number of the included conceptual studies (17 of the 36) 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 29 , 35 , 38 , 39 , 40 , 41 , 42 , 43 , 44 quoted the VH definition introduced by the SAGE working group, which may have led to an amplification of the SAGE definition. This may indicate that this definition is well recognized, but potentially overshadows less recognized conceptual definitions of VH. We chose to include all quoted definitions and found that many studies used more than one. We did not look further into conflicting definitions within the articles, but doing so could yield interesting insights.
In conclusion, we propose a definition of VH as a psychological state of indecisiveness that people may experience when making a decision regarding vaccination. We acknowledge that experiencing concerns, doubts or reluctance regarding vaccination may play a vital role in shaping VH. However, we argue that these factors have the highest potential to advance scientific knowledge when treated as relevant constructs integral to shaping VH, rather than treating them as synonymous to VH. Operationalizing VH by measuring or distinguishing subpopulations should ideally be directed at this state of indecision. To avoid confusion, it is important to separate VH from vaccination behaviour, which is already a well-defined concept. This proposal of a renewed definition of a concept that has been used for a decade could be perceived as ‘putting old wine in new bottles’. However, we feel that due to the large amount of highly varied literature, and given the importance of VH research in predicting, explaining and influencing immunization behaviour, it is necessary to take a snapshot of the status quo. The conclusion of this review is that VH is, for now, an impracticable concept, due to the confusing use of multiple, varied operationalizations. To aid further research, the VH concept must be clearly conceptualized and adapted from its broad and inclusive form to a pragmatic and refined alternative. Working on such an alternative, the field should first reach consensus on the definition and then measure VH accordingly. This approach allows for a much-needed comparison between studies to improve our understanding of VH determinants, correlates and consequences on an individual and societal level. Our way forward is to simplify and clarify the operationalization of VH by returning to its root core of indecisiveness.
This systematic review was registered on 11 November 2020 in the PROSPERO database (CRD42020211046). The record and study protocol are available at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=211046 .
Relevant publications were searched using the PubMed, Embase and PsycINFO databases to ensure coverage of all relevant research areas in the medical, public health and social science fields. The CINAHL database was also considered, but a pilot search revealed that its unique contributions were limited.
An experienced research librarian used the following keywords to develop a search strategy (Supplementary Methods ): ‘vaccination’, ‘immunization’, ‘vaccination refusal’, ‘vaccination avoidance’, ‘vaccination hesitation’, ‘vaccine hesitancy’, ‘vaccine uptake’, ‘vaccination behaviour’, ‘vaccination attitude’, ‘vaccine confidence’, ‘vaccine acceptance’ and ‘vaccine barriers’. The limitations included a publication date of between 2010 and the date of the search (14 January 2022). Conference abstracts were excluded from the search of the Embase database.
Eligibility criteria
The included studies were all published in peer-reviewed journals and written in English. All study types were eligible, except editorials and commentaries, as we sought to include original studies. Studies on animal vaccines were excluded.
The purpose of this review was to clarify the VH concept by analysing how it is operationalized. We recognized operationalizations at two main levels: conceptual and empirical. This resulted in three main groups: (1) studies describing or defining the VH concept and studies applying the concept by (2) identifying VH subpopulations and (3) measuring VH in individuals. This approach allowed comparison between conceptual and empirical operationalizations of VH.
Study selection
In the first selection round, two members of the research team used RAYYAN software to independently assess the titles and abstracts. Studies were selected when the title or abstract contained the term ‘vaccine hesitancy’. Studies were also selected if the title or abstract indicated that the full text contained further information on VH conceptualization, subpopulations or measurements. Papers without an abstract were selected for full-text screening. After double-screening, the results were de-blinded to allow the researchers to discuss their conflicting judgements until consensus was reached.
In the second selection round, the full texts were screened. The first 30% of studies were double-screened to establish a uniform method. Studies were screened on whether they met the criteria for one or more of the three categories (conceptualization, subpopulations and measurements). The category of ‘conceptualization’ included studies that describe, discuss or explore the VH concept or propose a novel VH measurement instrument. Studies falling into only the second category (subpopulations) were excluded if they merely distinguished between hesitant and non-hesitant groups, since a dichotomous grouping does not contribute to understanding of VH. The references from the included full-text articles were screened to find additional studies matching the selection criteria.
We deviated posthoc from our preregistered study protocol by adjusting the study selection criteria as follows. Initially, we also included studies containing the term ‘vaccine confidence’ (that is, with no mention or operationalization of vaccine hesitancy) as indicated in our study protocol. During the process, we realized that this deviated from our primary aim to clarify the VH concept by differentiating its related concepts. Therefore, we adapted the protocol and excluded 16 studies that were exclusively on vaccine confidence from our analysis
Data collection
The study characteristics were extracted from each of the full-text articles. Data were extracted by one researcher and verified by a second member of the research team. The variables included the first author, year of publication, research field of the first author, type of study, type of participants, number of participants, type of vaccination and country in which the study was conducted (with corresponding economic status) 447 . For the studies that do not include data collection, the country of origin was determined using the affiliation of the first author.
From the studies on VH conceptualization, text excerpts that define or describe VH or describe the relationship of VH to other concepts were extracted. These excerpts were further analysed in the qualitative analysis. From studies that describe different VH subpopulations, information about the categorization of these various subgroups was extracted, including the rationale for the distinguished subpopulations. From studies that describe VH measurements, the instrument(s) and criteria used to define VH were extracted.
Synthesis of results
The text excerpts extracted from the studies conceptualizing VH were thematically coded using ATLAS.ti software. Three research team members developed a coding book of themes and subthemes after independent coding of 30% of the studies. Thereafter, one researcher continued the coding process for the remaining studies. Any emerging new codes were discussed with the other research team members. The results were analysed qualitatively, and the predominant themes were identified by the three team members. When possible, results were grouped by research field and vaccine type to allow for comparison.
The data extracted from the studies describing VH subpopulations were summarized in a table and grouped according to the common themes identified. The data extracted from the studies describing a VH measurement were summarized in a table and grouped according to the instrument or method used. Where multiple measurement instruments are used in one study, the tool used to determine hesitancy was selected as the main instrument.
Quality assessment
The quality of each study was assessed using the MMAT 448 . This tool contains appraisal guidelines for different study types, covering the majority of the included studies. An overall score was calculated (1–5) on the basis of additional communication about the MMAT 2018 version, with higher scores indicating higher quality levels 449 . The first 20% of studies were assessed independently by two members of the research team to ensure consistency. Thereafter, one member of the research team continued the assessment.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data generated or analysed during this study are included in this article and its Supplementary Information . This systematic review is registered in PROSPERO (CRD42020211046).
Ten Threats to Global Health in 2019 (WHO, 2019); https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
Patel, M. K. et al. Progress toward regional measles elimination - worldwide, 2000–2019. MMWR Morb. Mortal. Wkly Rep. 69 , 1700–1705 (2020).
Article Google Scholar
Suijkerbuijk, A. W. et al. Economic costs of measles outbreak in the Netherlands, 2013–2014. Emerg. Infect. Dis. 21 , 2067–2069 (2015).
Ghebrehewet, S. et al. The economic cost of measles: healthcare, public health and societal costs of the 2012–13 outbreak in Merseyside, UK. Vaccine 34 , 1823–1831 (2016).
Chovatiya, R. & Silverberg, J. I. Inpatient morbidity and mortality of measles in the United States. PLoS ONE 15 , e0231329 (2020).
Article CAS Google Scholar
Feemster, K. A. & Szipszky, C. Resurgence of measles in the United States: how did we get here? Curr. Opin. Pediatr. 32 , 139–144 (2020).
Rechel, B., Richardson, E. & McKee, M. The Organization and Delivery of Vaccination Services in the European Union (The European Observatory on Health Systems and Policies, 2018). https://www.euro.who.int/__data/assets/pdf_file/0008/386684/vaccination-report-eng.pdf
Wilder-Smith, A. B. & Qureshi, K. Resurgence of measles in Europe: a systematic review on parental attitudes and beliefs of measles vaccine. J. Epidemiol. Glob. Health 10 , 46–58 (2020).
Bangura, J. B., Xiao, S., Qiu, D., Ouyang, F. & Chen, L. Barriers to childhood immunization in sub-Saharan Africa: a systematic review. BMC Public Health 20 , 1108 (2020).
Sweileh, W. M. Bibliometric analysis of global scientific literature on vaccine hesitancy in peer-reviewed journals (1990–2019). BMC Public Health 20 , 1252 (2020).
Frederiksen, L. S. F., Zhang, Y., Foged, C. & Thakur, A. The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front. Immunol. 11 , 1817 (2020).
Randolph, H. E. & Barreiro, L. B. Herd immunity: understanding COVID-19. Immunity 52 , 737–741 (2020).
Dror, A. A. et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 35 , 775–779 (2020).
MacDonald, N. E. Vaccine hesitancy: definition, scope and determinants. Vaccine 33 , 4161–4164 (2015).
Podsakoff, P. M., MacKenzie, S. B. & Podsakoff, N. P. Recommendations for creating better concept definitions in the organizational, behavioral, and social sciences. Organ. Res. Methods 19 , 159–203 (2016).
Shapiro, G. K. et al. A critical review of measures of childhood vaccine confidence. Curr. Opin. Immunol. 71 , 34–45 (2021).
Brewer, N. T., Chapman, G. B., Rothman, A. J., Leask, J. & Kempe, A. Increasing vaccination: putting psychological science into action. Psychol. Sci. Public Interest 18 , 149–207 (2017).
Bedford, H. et al. Vaccine hesitancy, refusal and access barriers: the need for clarity in terminology. Vaccine 36 , 6556–6558 (2018).
Orenstein, W. et al. Assessing the state of vaccine confidence in the United States: recommendations from the national vaccine advisory committee. Public Health Rep. 130 , 573–595 (2015).
Schmid, P., Rauber, D., Betsch, C., Lidolt, G. & Denker, M. L. Barriers of influenza vaccination intention and behavior - a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS ONE 12 , e0170550 (2017).
Dubé, E. et al. “Nature does things well, why should we interfere?”: vaccine hesitancy among mothers. Qual. Health Res. 26 , 411–425 (2016).
Betsch, C. et al. Beyond confidence: development of a measure assessing the 5C psychological antecedents of vaccination. PLoS ONE 13 , e0208601 (2018).
Peretti-Watel, P., Larson, H. J., Ward, J. K., Schulz, W. S. & Verger, P. Vaccine hesitancy: clarifying a theoretical framework for an ambiguous notion. PLoS Curr. https://doi.org/10.1371/currents.outbreaks.6844c80ff9f5b273f34c91f71b7fc289 (2015).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Brit. Med. J. 372 , n71 (2021).
Bertoncello, C. et al. Socioeconomic determinants in vaccine hesitancy and vaccine refusal in Italy. Vaccines 8 , 276 (2020).
Deml, M. J. et al. ‘Problem patients and physicians’ failures’: what it means for doctors to counsel vaccine hesitant patients in Switzerland. Soc. Sci. Med. 255 , 112946 (2020).
Dubé, E. et al. Vaccine hesitancy: an overview. Hum. Vaccin. Immunother. 9 , 1763–1773 (2013).
Dubé, E., Gagnon, D., Nickels, E., Jeram, S. & Schuster, M. Mapping vaccine hesitancy–country-specific characteristics of a global phenomenon. Vaccine 32 , 6649–6654 (2014).
Dubé, E. et al. Understanding vaccine hesitancy in Canada: results of a consultation study by the Canadian Immunization Research Network. PLoS ONE 11 , e0156118 (2016).
Gowda, C. & Dempsey, A. F. The rise (and fall?) of parental vaccine hesitancy. Hum. Vaccin. Immunother. 9 , 1755–1762 (2013).
Kestenbaum, L. A. & Feemster, K. A. Identifying and addressing vaccine hesitancy. Pediatr. Ann. 44 , e71–e75 (2015).
Larson, H. J. Negotiating vaccine acceptance in an era of reluctance. Hum. Vaccin. Immunother. 9 , 1779–1781 (2013).
Oduwole, E. O., Pienaar, E. D., Mahomed, H. & Wiysonge, C. S. Current tools available for investigating vaccine hesitancy: a scoping review protocol. BMJ Open 9 , e033245 (2019).
Opel, D. J. et al. Development of a survey to identify vaccine-hesitant parents: the parent attitudes about childhood vaccines survey. Hum. Vaccin. 7 , 419–425 (2011).
Smith, M. J. Promoting vaccine confidence. Infect. Dis. Clin. North Am. 29 , 759–769 (2015).
Dubé, È., Ward, J. K., Verger, P. & MacDonald, N. E. Vaccine hesitancy, acceptance, and anti-vaccination: trends and future prospects for public health. Annu Rev. Public Health 42 , 175–191 (2021).
Popper-Giveon, A. & Keshet, Y. Non-Vaccination Stage Model (NVST): the decision-making process among Israeli ultra-orthodox Jewish parents. Health https://doi.org/10.1177/1363459320988884 (2021).
Kumar, D., Chandra, R., Mathur, M., Samdariya, S. & Kapoor, N. Vaccine hesitancy: understanding better to address better. Isr. J. Health Policy Res . 5 , 2 (2016).
McIntosh, E. D., Janda, J., Ehrich, J. H., Pettoello-Mantovani, M. & Somekh, E. Vaccine hesitancy and refusal. J. Pediatr. 175 , 248–249.e1 (2016).
Díaz Crescitelli, M. E. et al. A meta-synthesis study of the key elements involved in childhood vaccine hesitancy. Public Health 180 , 38–45 (2020).
Larson, H. J. et al. Measuring vaccine hesitancy: the development of a survey tool. Vaccine 33 , 4165–4175 (2015).
Howard, M. C. A more comprehensive measure of vaccine hesitancy: creation of the Multidimensional Vaccine Hesitancy Scale (MVHS). J. Health Psychol . https://doi.org/10.1177/13591053211042062 (2021).
Turner, P. J., Larson, H., Dubé, È. & Fisher, A. Vaccine hesitancy: drivers and how the allergy community can help. J. Allergy Clin. Immunol. Pract. 9 , 3568–3574 (2021).
Freeman, D. et al. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol. Med . https://doi.org/10.1017/s0033291720005188 (2020).
Su, Z. et al. A race for a better understanding of COVID-19 vaccine non-adopters. Brain Behav. Immun. Health 9 , 100159 (2020).
Sarathchandra, D., Navin, M. C., Largent, M. A. & McCright, A. M. A survey instrument for measuring vaccine acceptance. Prev. Med. 109 , 1–7 (2018).
Leask, J. et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 12 , 154 (2012).
Jankovic, S. Childhood vaccination in the twenty-first century: parental concerns and challenges for physicians. Arh. Farm. 69 , 452–468 (2019).
Hagood, E. A. & Mintzer Herlihy, S. Addressing heterogeneous parental concerns about vaccination with a multiple-source model: a parent and educator perspective. Hum. Vaccin. Immunother. 9 , 1790–1794 (2013).
Blaisdell, L. L., Gutheil, C., Hootsmans, N. A. & Han, P. K. Unknown risks: parental hesitation about vaccination. Med. Decis. Mak. 36 , 479–489 (2016).
Amin, A. B. et al. Association of moral values with vaccine hesitancy. Nat. Hum. Behav. 1 , 873–880 (2017).
Kotta, I., Kalcza-Janosi, K., Szabo, K. & Marschalko, E. E. Development and validation of the multidimensional COVID-19 vaccine hesitancy scale. Hum. Vaccin. Immunother. 18 , 1–10 (2021).
Sjögren, E., Ask, L. S., Örtqvist, Å. & Asp, M. Parental conceptions of the rotavirus vaccine during implementation in Stockholm: a phenomenographic study. J. Child Health Care 21 , 476–487 (2017).
Frew, P. M. et al. Development of a US trust measure to assess and monitor parental confidence in the vaccine system. Vaccine 37 , 325–332 (2019).
Smith, J. C., Appleton, M. & MacDonald, N. E. Building confidence in vaccines. Adv. Exp. Med. Biol. 764 , 81–98 (2013).
Larson, H. J., Schulz, W. S., Tucker, J. D. & Smith, D. M. Measuring vaccine confidence: introducing a global vaccine confidence index. PLoS Curr. https://doi.org/10.1371/currents.outbreaks.ce0f6177bc97332602a8e3fe7d7f7cc4 (2015).
Ramanadhan, S., Galarce, E., Xuan, Z., Alexander-Molloy, J. & Viswanath, K. Addressing the vaccine hesitancy continuum: an audience segmentation analysis of american adults who did not receive the 2009 H1N1 vaccine. Vaccines 3 , 556–578 (2015).
Rossen, I., Hurlstone, M. J., Dunlop, P. D. & Lawrence, C. Accepters, fence sitters, or rejecters: moral profiles of vaccination attitudes. Soc. Sci. Med. 224 , 23–27 (2019).
Rozbroj, T., Lyons, A. & Lucke, J. Vaccine-hesitant and vaccine-refusing parents’ reflections on the way parenthood changed their attitudes to vaccination. J. Community Health 45 , 63–72 (2020).
Schwartz, J. L. & Caplan, A. L. Vaccination refusal: ethics, individual rights, and the common good. Prim. Care 38 , 717–728 (2011).
Shay, L. A. et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 141 , e20172312 (2018).
Oladejo, O. et al. Comparative analysis of the Parent Attitudes about Childhood Vaccines (PACV) short scale and the five categories of vaccine acceptance identified by Gust et al. Vaccine 34 , 4964–4968 (2016).
Bouchez, M. et al. Physicians’ decision processes about the HPV vaccine: a qualitative study. Vaccine 39 , 521–528 (2021).
Elwy, A. R. et al. Vaccine hesitancy as an opportunity for engagement: a rapid qualitative study of patients and employees in the U.S. Veterans Affairs healthcare system. Vaccine 9 , 100116 (2021).
CAS Google Scholar
Paris, C. et al. COVID-19 vaccine hesitancy among healthcare workers. Infect. Dis. Now 51 , 484–487 (2021).
Tram, K. H. et al. Deliberation, dissent, and distrust: understanding distinct drivers of COVID-19 vaccine hesitancy in the United States. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciab633 (2021).
Vulpe, S. N. & Rughiniş, C. Social amplification of risk and “probable vaccine damage”: a typology of vaccination beliefs in 28 European countries. Vaccine 39 , 1508–1515 (2021).
Wang, M. W. et al. COVID-19 vaccination acceptance among healthcare workers and non-healthcare workers in china: a survey. Front. Public Health 9 , 709056 (2021).
Shapiro, G. K. et al. Using an integrated conceptual framework to investigate parents’ HPV vaccine decision for their daughters and sons. Prev. Med. 116 , 203–210 (2018).
Tatar, O., Shapiro, G. K., Perez, S., Wade, K. & Rosberger, Z. Using the precaution adoption process model to clarify human papillomavirus vaccine hesitancy in Canadian parents of girls and parents of boys. Hum. Vaccin. Immunother. 15 , 1803–1814 (2019).
Dubov, A. et al. Predictors of COVID-19 vaccine acceptance and hesitancy among healthcare workers in Southern California: not just “anti” vs. “pro” vaccine. Vaccines 9 , 1428 (2021).
Brunelli, L. et al. Parental trust and beliefs after the discovery of a six-year-long failure to vaccinate. Hum. Vaccin. Immunother. 17 , 583–587 (2020).
Armiento, R. et al. Impact of Australian mandatory ‘No Jab, No Pay’ and ‘No Jab, No Play’ immunisation policies on immunisation services, parental attitudes to vaccination and vaccine uptake, in a tertiary paediatric hospital, the Royal Children’s Hospital, Melbourne. Vaccine 38 , 5231–5240 (2020).
Berry, N. J. et al. Sharing knowledge about immunisation (SKAI): an exploration of parents’ communication needs to inform development of a clinical communication support intervention. Vaccine 36 , 6480–6490 (2018).
Bocquier, A. et al. Social differentiation of vaccine hesitancy among French parents and the mediating role of trust and commitment to health: a nationwide cross-sectional study. Vaccine 36 , 7666–7673 (2018).
Chang, K. & Lee, S. Y. Why do some Korean parents hesitate to vaccinate their children? Epidemiol. Health 41 , e2019031 (2019).
Danchin, M. H. et al. Vaccine decision-making begins in pregnancy: correlation between vaccine concerns, intentions and maternal vaccination with subsequent childhood vaccine uptake. Vaccine 36 , 6473–6479 (2018).
Forbes, T. A., McMinn, A., Crawford, N., Leask, J. & Danchin, M. Vaccination uptake by vaccine-hesitant parents attending a specialist immunization clinic in Australia. Hum. Vaccin. Immunother. 11 , 2895–2903 (2015).
Glanternik, J. R. et al. Evaluation of a vaccine-communication tool for physicians. J. Pediatr. 224 , 72–78.e1 (2020).
McDonald, P. et al. Exploring California’s new law eliminating personal belief exemptions to childhood vaccines and vaccine decision-making among homeschooling mothers in California. Vaccine 37 , 742–750 (2019).
Noyman-Veksler, G., Greenberg, D., Grotto, I. & Shahar, G. Parents’ malevolent personification of mass vaccination solidifies vaccine hesitancy. J. Health Psychol . https://doi.org/10.1177/1359105320903475 (2020).
Duong, M. C., Nguyen, H. T. & Duong, M. Evaluating COVID-19 vaccine hesitancy: a qualitative study from Vietnam. Diabetes Metab. Syndr. 16 , 102363 (2022).
Gatto, N. M. et al. Correlates of COVID-19 vaccine acceptance, hesitancy and refusal among employees of a safety net California County health system with an early and aggressive vaccination program: results from a cross-sectional survey. Vaccines 9 , 1152 (2021).
Moore, J. X. et al. Correlates of COVID-19 vaccine hesitancy among a community sample of African Americans living in the southern United States. Vaccines 9 , 879 (2021).
Murphy, J. et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 12 , 29 (2021).
Musa, S. et al. A qualitative interview study with parents to identify barriers and drivers to childhood vaccination and inform public health interventions. Hum. Vaccin. Immunother. 17 , 3023–3033 (2021).
Steffens, M. S., Bullivant, B., Bolsewicz, K. T., King, C. & Beard, F. “to protect myself, my friends, family, workmates and patients… and to play my part”: COVID-19 vaccination perceptions among health and aged care workers in New South Wales, Australia. Int. J. Environ. Res. Public Health 18 , 8954 (2021).
Tavolacci, M. P., Dechelotte, P. & Ladner, J. COVID-19 vaccine acceptance, hesitancy, and resistancy among university students in France. Vaccines 9 , 654 (2021).
Buttenheim, A. M. et al. Vaccine exemption requirements and parental vaccine attitudes: an online experiment. Vaccine 38 , 2620–2625 (2020).
Corben, P. & Leask, J. Vaccination hesitancy in the antenatal period: a cross-sectional survey. BMC Public Health 18 , 566 (2018).
Gagneur, A. et al. Promoting vaccination in maternity wards ─ motivational interview technique reduces hesitancy and enhances intention to vaccinate, results from a multicentre non-controlled pre- and post-intervention RCT-nested study, Quebec, March 2014 to February 2015. Eur. Surveill. 24 , 1800641 (2019).
Lau, L. H. W., Lee, S. S. & Wong, N. S. The continuum of influenza vaccine hesitancy among nursing professionals in Hong Kong. Vaccine 38 , 6785–6793 (2020).
Nekrasova, E. et al. Vaccine hesitancy and influenza beliefs among parents of children requiring a second dose of influenza vaccine in a season: an American Academy of Pediatrics (AAP) Pediatric Research in Office Settings (PROS) study. Hum. Vaccin. Immunother. 16 , 1070–1077 (2020).
Verger, P. et al. Prevalence and correlates of vaccine hesitancy among general practitioners: a cross-sectional telephone survey in France, April to July 2014. Eur. Surveill. 21 , 30406 (2016).
Bianco, A. et al. Parental COVID-19 vaccine hesitancy: a cross-sectional survey in Italy. Expert Rev. Vaccines 21 , 541–547 (2022).
Edwards, B., Biddle, N., Gray, M. & Sollis, K. COVID-19 vaccine hesitancy and resistance: correlates in a nationally representative longitudinal survey of the Australian population. PLoS ONE 16 , e0248892 (2021).
Hsieh, Y. L., Rak, S., SteelFisher, G. K. & Bauhoff, S. Effect of the suspension of the J&J COVID-19 vaccine on vaccine hesitancy in the United States. Vaccine 40 , 424–427 (2022).
Umakanthan, S., Patil, S., Subramaniam, N. & Sharma, R. COVID-19 vaccine hesitancy and resistance in India explored through a population-based longitudinal survey. Vaccines 9 , 1064 (2021).
Zheng, W. et al. COVID-19 vaccine uptake and hesitancy among HIV-infected men who have sex with men in mainland China: a cross-sectional survey. Hum. Vaccin. Immunother. 17 , 4971–4981 (2021).
Baniak, L. M., Luyster, F. S., Raible, C. A., McCray, E. E. & Strollo, P. J. COVID-19 vaccine hesitancy and uptake among nursing staff during an active vaccine rollout. Vaccines 9 , 858 (2021).
Bou Hamdan, M., Singh, S., Polavarapu, M., Jordan, T. R. & Melhem, N. M. COVID-19 vaccine hesitancy among university students in Lebanon. Epidemiol. Infect. 149 , e242 (2021).
Castellano-Tejedor, C., Torres-Serrano, M. & Cencerrado, A. Unveiling Associations of COVID-19 vaccine acceptance, hesitancy, and resistance: a cross-sectional community-based adult survey. Int. J. Environ. Res. Public Health 18 , 12348 (2021).
Freeman, D. et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health 6 , e416–e427 (2021).
Janssen, C. et al. Hesitancy towards COVID-19 vaccination among healthcare workers: a multi-centric survey in France. Vaccines 9 , 547 (2021).
Khaled, S. M. et al. Prevalence and potential determinants of COVID-19 vaccine hesitancy and resistance in Qatar: results from a nationally representative survey of Qatari nationals and migrants between December 2020 and January 2021. Vaccines 9 , 471 (2021).
Montagni, I. et al. Acceptance of a Covid-19 vaccine is associated with ability to detect fake news and health literacy. J. Public Health https://doi.org/10.1093/pubmed/fdab028 (2021).
Qunaibi, E., Basheti, I., Soudy, M. & Sultan, I. Hesitancy of Arab healthcare workers towards COVID-19 vaccination: a large-scale multinational study. Vaccines 9 , 446 (2021).
Reno, C. et al. Enhancing COVID-19 vaccines acceptance: results from a survey on vaccine hesitancy in northern Italy. Vaccines 9 , 378 (2021).
Rodríguez-Blanco, N. et al. Willingness to be vaccinated against COVID-19 in Spain before the start of vaccination: a cross-sectional study. Int. J. Environ. Res. Public Health 18 , 5272 (2021).
Leung, C. L. K. et al. Profiling vaccine believers and skeptics in nurses: a latent profile analysis. Int. J. Nurs. Stud. 126 , 104142 (2021).
Salerno, L., Craxì, L., Amodio, E. & Lo Coco, G. Factors affecting hesitancy to mRNA and viral vector COVID-19 vaccines among college students in Italy. Vaccines 9 , 927 (2021).
Zona, S. et al. Anti-COVID vaccination for adolescents: a survey on determinants of vaccine parental hesitancy. Vaccines 9 , 1309 (2021).
Alabbad, A. A., Alsaad, A. K., Al Shaalan, M. A., Alola, S. & Albanyan, E. A. Prevalence of influenza vaccine hesitancy at a tertiary care hospital in Riyadh, Saudi Arabia. J. Infect. Public Health 11 , 491–499 (2018).
Barello, S., Nania, T., Dellafiore, F., Graffigna, G. & Caruso, R. ‘Vaccine hesitancy’ among university students in Italy during the COVID-19 pandemic. Eur. J. Epidemiol. 35 , 781–783 (2020).
Bogart, L. M. et al. COVID-19 related medical mistrust, health impacts, and potential vaccine hesitancy among Black Americans living with HIV. J. Acquir. Immune Defic. Syndr. 86 , 200–207 (2020).
Brown, A. L. et al. Vaccine confidence and hesitancy in Brazil. Cad. Saude Publica 34 , e00011618 (2018).
Byström, E., Lindstrand, A., Bergström, J., Riesbeck, K. & Roth, A. Confidence in the National Immunization Program among parents in Sweden 2016 – a cross-sectional survey. Vaccine 38 , 3909–3917 (2020).
Costantino, C. et al. Determinants of vaccine hesitancy and effectiveness of vaccination counseling interventions among a sample of the general population in Palermo, Italy. Hum. Vaccin. Immunother. 16 , 2415–2421 (2020).
Deas, J., Bean, S. J., Sokolovska, I. & Fautin, C. Childhood vaccine attitudes and information sources among Oregon parents and guardians. Health Promot. Pract. 20 , 529–538 (2019).
Dempsey, A. F., Pyrzanowski, J., Campagna, E. J., Lockhart, S. & O’Leary, S. T. Parent report of provider HPV vaccine communication strategies used during a randomized, controlled trial of a provider communication intervention. Vaccine 37 , 1307–1312 (2019).
Detoc, M. et al. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine 38 , 7002–7006 (2020).
Du, F. et al. The determinants of vaccine hesitancy in China: a cross-sectional study following the Changchun Changsheng vaccine incident. Vaccine 38 , 7464–7471 (2020).
Dubé, E., Gagnon, D., Zhou, Z. & Deceuninck, G. Parental vaccine hesitancy in Quebec (Canada). PLoS Curr . https://doi.org/10.1371/currents.outbreaks.9e239605f4d320c6ad27ce2aea5aaad2 (2016).
Fisher, K. A. et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann. Intern. Med. 173 , 964–973 (2020).
Gesser-Edelsburg, A., Walter, N., Shir-Raz, Y., Sassoni Bar-Lev, O. & Rosenblat, S. The behind-the-scenes activity of parental decision-making discourse regarding childhood vaccination. Am. J. Infect. Control 45 , 267–271 (2017).
Gowda, C., Schaffer, S. E., Kopec, K., Markel, A. & Dempsey, A. F. A pilot study on the effects of individually tailored education for MMR vaccine-hesitant parents on MMR vaccination intention. Hum. Vaccin. Immunother. 9 , 437–445 (2013).
Gowda, C., Schaffer, S. E., Kopec, K., Markel, A. & Dempsey, A. F. Does the relative importance of MMR vaccine concerns differ by degree of parental vaccine hesitancy?: an exploratory study. Hum. Vaccin. Immunother. 9 , 430–436 (2013).
Hadjipanayis, A. et al. Vaccine confidence among parents: large scale study in eighteen European countries. Vaccine 38 , 1505–1512 (2020).
Hirth, J. M., Fuchs, E. L., Chang, M., Fernandez, M. E. & Berenson, A. B. Variations in reason for intention not to vaccinate across time, region, and by race/ethnicity, NIS-Teen (2008–2016). Vaccine 37 , 595–601 (2019).
Karlsson, L. C. et al. The association between vaccination confidence, vaccination behavior, and willingness to recommend vaccines among Finnish healthcare workers. PLoS ONE 14 , e0224330 (2019).
Khodadadi, A. B., Redden, D. T. & Scarinci, I. C. HPV vaccination hesitancy among latina immigrant mothers despite physician recommendation. Ethn. Dis. 30 , 661–670 (2020).
Melot, B. et al. Knowledge, attitudes and practices about vaccination in Trentino, Italy in 2019. Hum. Vaccin. Immunother. 17 , 259–268 (2020).
Mereu, N. et al. Vaccination attitude and communication in early settings: an exploratory study. Vaccines 8 , 701 (2020).
Opel, D. J. et al. Characterizing providers’ immunization communication practices during health supervision visits with vaccine-hesitant parents: a pilot study. Vaccine 30 , 1269–1275 (2012).
Özceylan, G., Toprak, D. & Esen, E. S. Vaccine rejection and hesitation in Turkey. Hum. Vaccin. Immunother. 16 , 1034–1039 (2020).
Pahud, B. et al. A randomized controlled trial of an online immunization curriculum. Vaccine 38 , 7299–7307 (2020).
Quinn, S. C. et al. Breaking down the monolith: understanding flu vaccine uptake among African Americans. SSM Popul. Health 4 , 25–36 (2018).
Quinn, S. C. et al. Exploring racial influences on flu vaccine attitudes and behavior: results of a national survey of White and African American adults. Vaccine 35 , 1167–1174 (2017).
Quinn, S. C., Jamison, A. M., An, J., Hancock, G. R. & Freimuth, V. S. Measuring vaccine hesitancy, confidence, trust and flu vaccine uptake: results of a national survey of White and African American adults. Vaccine 37 , 1168–1173 (2019).
Repalust, A., Šević, S., Rihtar, S. & Štulhofer, A. Childhood vaccine refusal and hesitancy intentions in Croatia: insights from a population-based study. Psychol. Health Med. 22 , 1045–1055 (2017).
Rey, D. et al. Vaccine hesitancy in the French population in 2016, and its association with vaccine uptake and perceived vaccine risk-benefit balance. Eur. Surveill. 23 , 17–00816 (2018).
Santibanez, T. A. et al. Parental vaccine hesitancy and childhood influenza vaccination. Pediatrics 146 , e2020007609 (2020).
Schollin Ask, L. et al. Receiving early information and trusting Swedish child health centre nurses increased parents’ willingness to vaccinate against rotavirus infections. Acta Paediatr. 106 , 1309–1316 (2017).
Suryadevara, M., Handel, A., Bonville, C. A., Cibula, D. A. & Domachowske, J. B. Pediatric provider vaccine hesitancy: an under-recognized obstacle to immunizing children. Vaccine 33 , 6629–6634 (2015).
Taylor, S. et al. A proactive approach for managing COVID-19: the importance of understanding the motivational roots of vaccination hesitancy for SARS-CoV2. Front. Psychol. 11 , 575950 (2020).
Tran, B. X. et al. Media representation of vaccine side effects and its impact on utilization of vaccination services in Vietnam. Patient Prefer. Adherence 12 , 1717–1728 (2018).
Wilson, R. et al. Vaccine hesitancy and self-vaccination behaviors among nurses in southeastern France. Vaccine 38 , 1144–1151 (2020).
Yu, W. et al. Two media-reported vaccine events in China from 2013 to 2016: impact on confidence and vaccine utilization. Vaccine 38 , 5541–5547 (2020).
Abdel-Qader, D. H. et al. Pharmacists-physicians collaborative intervention to reduce vaccine hesitancy and resistance: a randomized controlled trial. Vaccine X 10 , 100135 (2022).
Abedin, M. et al. Willingness to vaccinate against COVID-19 among Bangladeshi adults: understanding the strategies to optimize vaccination coverage. PLoS ONE 16 , e0250495 (2021).
Abou Leila, R., Salamah, M. & El-Nigoumi, S. Reducing COVID-19 vaccine hesitancy by implementing organizational intervention in a primary care setting in Bahrain. Cureus 13 , e19282 (2021).
Google Scholar
Aguilar Ticona, J. P. et al. Willingness to get the COVID-19 vaccine among residents of slum settlements. Vaccines 9 , 951 (2021).
Ahmad, K. K. Coronavirus disease 2019 vaccine hesitancy in the Kurdistan region: a cross-sectional national survey. Arch. Razi Inst. 76 , 751–759 (2021).
Al-Ayyadhi, N., Ramadan, M. M., Al-Tayar, E., Al-Mathkouri, R. & Al-Awadhi, S. Determinants of hesitancy towards COVID-19 vaccines in State of Kuwait: an exploratory internet-based survey. Risk Manage. Healthc. Policy 14 , 4967–4981 (2021).
Alfieri, N. L. et al. Parental COVID-19 vaccine hesitancy for children: vulnerability in an urban hotspot. BMC Public Health 21 , 1662 (2021).
Alibrahim, J. & Awad, A. COVID-19 vaccine hesitancy among the public in Kuwait: a cross-sectional survey. Int. J. Environ. Res. Public Health 18 , 8836 (2021).
Allington, D., McAndrew, S., Moxham-Hall, V. & Duffy, B. Coronavirus conspiracy suspicions, general vaccine attitudes, trust and coronavirus information source as predictors of vaccine hesitancy among UK residents during the COVID-19 pandemic. Psychol. Med . https://doi.org/10.1017/s0033291721001434 (2021).
Al-Sanafi, M. & Sallam, M. Psychological determinants of COVID-19 vaccine acceptance among healthcare workers in Kuwait: a cross-sectional study using the 5C and vaccine conspiracy beliefs scales. Vaccines 9 , 701 (2021).
Alshahrani, S. M. et al. Acceptability of COVID-19 vaccination in Saudi Arabia: a cross-sectional study using a web-based survey. Hum. Vaccin. Immunother. 17 , 3338–3347 (2021).
Al-Wutayd, O., Khalil, R. & Rajar, A. B. Sociodemographic and behavioral predictors of COVID-19 vaccine hesitancy in Pakistan. J. Multidiscip. Healthc. 14 , 2847–2856 (2021).
Alzeer, A. A. et al. The influence of demographics on influenza vaccine awareness and hesitancy among adults visiting educational hospital in Saudi Arabia. Saudi Pharm. J. 29 , 188–193 (2021).
Alzubaidi, H. et al. A mixed-methods study to assess COVID-19 vaccination acceptability among university students in the United Arab Emirates. Hum. Vaccin. Immunother. 17 , 4074–4082 (2021).
Anandraj, J., Krishnamoorthy, Y., Sivanantham, P., Gnanadas, J. & Kar, S. S. Impact of second wave of COVID-19 pandemic on the hesitancy and refusal of COVID-19 vaccination in Puducherry, India: a longitudinal study. Hum. Vaccin. Immunother. 17 , 5024–5029 (2021).
Arvanitis, M. et al. Factors associated with COVID-19 vaccine trust and hesitancy among adults with chronic conditions. Prev. Med. Rep. 24 , 101484 (2021).
Baccolini, V. et al. COVID-19 vaccine hesitancy among Italian university students: a cross-sectional survey during the first months of the vaccination campaign. Vaccines 9 , 1292 (2021).
Bacon, A. M. & Taylor, S. Vaccination hesitancy and conspiracy beliefs in the UK During the SARS-COV-2 (COVID-19) pandemic. Int. J. Behav. Med. 29 , 448–455 (2022).
Badr, H. et al. Overcoming COVID-19 vaccine hesitancy: insights from an online population-based survey in the United States. Vaccines 9 , 1100 (2021).
Batty, G. D., Deary, I. J., Fawns-Ritchie, C., Gale, C. R. & Altschul, D. Pre-pandemic cognitive function and COVID-19 vaccine hesitancy: cohort study. Brain Behav. Immun. 96 , 100–105 (2021).
Benham, J. L. et al. COVID-19 vaccine-related attitudes and beliefs in Canada: national cross-sectional survey and cluster analysis. JMIR Public Health Surveill. 7 , e30424 (2021).
Bolatov, A. K., Seisembekov, T. Z., Askarova, A. Z. & Pavalkis, D. Barriers to COVID-19 vaccination among medical students in Kazakhstan: development, validation, and use of a new COVID-19 vaccine hesitancy scale. Hum. Vaccin. Immunother. 17 , 4982–4992 (2021).
Butter, S., McGlinchey, E., Berry, E. & Armour, C. Psychological, social, and situational factors associated with COVID-19 vaccination intentions: A study of UK key workers and non-key workers. Br. J. Health Psychol. 27 , 13–29 (2022).
Chandani, S. et al. COVID-19 vaccination hesitancy in India: state of the nation and priorities for research. Brain Behav. Immun. Health 18 , 100375 (2021).
Chaudhary, F. A. et al. Factors influencing COVID-19 vaccine hesitancy and acceptance among the Pakistani population. Hum. Vaccin. Immunother. 17 , 3365–3370 (2021).
Chen, H. et al. Health belief model perspective on the control of COVID-19 vaccine hesitancy and the promotion of vaccination in China: web-based cross-sectional study. J. Med. Internet Res. 23 , e29329 (2021).
Cherian, V., Saini, N. K., Sharma, A. K. & Philip, J. Prevalence and predictors of vaccine hesitancy in an urbanized agglomeration of New Delhi, India. J. Public Health 44 , 70–76 (2022).
Contoli, B. et al. What is the willingness to receive vaccination against COVID-19 among the elderly in Italy? Data from the PASSI d’Argento surveillance system. Front. Public Health 9 , 736976 (2021).
Cordina, M., Lauri, M. A. & Lauri, J. Attitudes towards COVID-19 vaccination, vaccine hesitancy and intention to take the vaccine. Pharm. Pract. 19 , 2317 (2021).
Costantino, A. et al. COVID-19 vaccine acceptance among liver transplant recipients. Vaccines 9 , 1314 (2021).
Costantino, A. et al. COVID-19 vaccine: a survey of hesitancy in patients with celiac disease. Vaccines 9 , 511 (2021).
Coughenour, C., Gakh, M., Sharma, M., Labus, B. & Chien, L. C. Assessing determinants of COVID-19 vaccine hesitancy in Nevada. Health Secur. 19 , 592–604 (2021).
Crane, M. A., Faden, R. R. & Romley, J. A. Disparities in county COVID-19 vaccination rates linked to disadvantage and hesitancy. Health Aff. 40 , 1792–1796 (2021).
Danabal, K. G. M., Magesh, S. S., Saravanan, S. & Gopichandran, V. Attitude towards COVID 19 vaccines and vaccine hesitancy in urban and rural communities in Tamil Nadu, India - a community based survey. BMC Health Serv. Res. 21 , 994 (2021).
Doherty, I. A. et al. COVID-19 vaccine hesitancy in underserved communities of North Carolina. PLoS ONE 16 , e0248542 (2021).
Du, F. et al. Access to vaccination information and confidence/hesitancy towards childhood vaccination: a cross-sectional survey in China. Vaccines 9 , 201 (2021).
Du, M., Tao, L. & Liu, J. The association between risk perception and COVID-19 vaccine hesitancy for children among reproductive women in China: an online survey. Front. Med. 8 , 741298 (2021).
Ebrahimi, O. V. et al. Risk, trust, and flawed assumptions: vaccine hesitancy during the COVID-19 pandemic. Front. Public Health 9 , 700213 (2021).
Ehde, D. M., Roberts, M. K., Humbert, A. T., Herring, T. E. & Alschuler, K. N. COVID-19 vaccine hesitancy in adults with multiple sclerosis in the United States: a follow up survey during the initial vaccine rollout in 2021. Mult. Scler. Relat. Disord. 54 , 103163 (2021).
Ekstrand, M. L. et al. COVID-19 vaccine hesitancy among PLWH in South India: implications for vaccination campaigns. J. Acquir. Immune Defic. Syndr. 88 , 421–425 (2021).
Elizondo-Alzola, U. et al. Vaccine hesitancy among paediatric nurses: prevalence and associated factors. PLoS ONE 16 , e0251735 (2021).
Emerson, E. et al. Vaccine hesitancy among working-age adults with/without disability in the UK. Public Health 200 , 106–108 (2021).
Epstein, S. et al. Vaccination Against SARS-CoV-2 in neuroinflammatory disease: early safety/tolerability data. Mult. Scler. Relat. Disord. 57 , 103433 (2021).
Eyllon, M. et al. Associations between psychiatric morbidity and COVID-19 vaccine hesitancy: an analysis of electronic health records and patient survey. Psychiatry Res. 307 , 114329 (2022).
Fares, S., Elmnyer, M. M., Mohamed, S. S. & Elsayed, R. COVID-19 vaccination perception and attitude among healthcare workers in Egypt. J. Prim. Care Community Health 12 , 21501327211013303 (2021).
Fayed, A. A. et al. Willingness to receive the COVID-19 and seasonal influenza vaccines among the Saudi population and vaccine uptake during the initial stage of the national vaccination campaign: a cross-sectional survey. Vaccines 9 , 765 (2021).
Fazel, M. et al. Willingness of children and adolescents to have a COVID-19 vaccination: results of a large whole schools survey in England. eClinicalMedicine 40 , 101144 (2021).
Fernández-Penny, F. E. et al. COVID-19 vaccine hesitancy among patients in two urban emergency departments. Acad. Emerg. Med . 28 , 1100–1107 (2021).
Fisher, K. A. et al. Preferences for COVID-19 vaccination information and location: associations with vaccine hesitancy, race and ethnicity. Vaccine 39 , 6591–6594 (2021).
Gatwood, J., McKnight, M., Fiscus, M., Hohmeier, K. C. & Chisholm-Burns, M. Factors influencing likelihood of COVID-19 vaccination: a survey of Tennessee adults. Am. J. Health Syst. Pharm. 78 , 879–889 (2021).
Gbeasor-Komlanvi, F. A. et al. Prevalence and factors associated with COVID-19 vaccine hesitancy in health professionals in Togo, 2021. Public Health Pract. 2 , 100220 (2021).
Gendler, Y. & Ofri, L. Investigating the influence of vaccine literacy, vaccine perception and vaccine hesitancy on Israeli parents’ acceptance of the COVID-19 vaccine for their children: a cross-sectional study. Vaccines 9 (2021).
Gentile, A. et al. Vaccine hesitancy in Argentina: validation of WHO scale for parents. Vaccine 39 , 4611–4619 (2021).
Gerretsen, P. et al. Individual determinants of COVID-19 vaccine hesitancy. PLoS ONE 16 , e0258462 (2021).
Gerretsen, P. et al. Vaccine hesitancy is a barrier to achieving equitable herd immunity among racial minorities. Front. Med. 8 , 668299 (2021).
Griva, K. et al. Evaluating rRates and determinants of COVID-19 vaccine hesitancy for adults and children in the Singapore population: Strengthening Our Community’s Resilience against Threats from Emerging Infections (SOCRATEs) Cohort. Vaccines 9 , 1415 (2021).
Guaraldi, F. et al. Rate and predictors of hesitancy toward SARS-CoV-2 vaccine among type 2 diabetic patients: results from an Italian survey. Vaccines 9 , 460 (2021).
Hara, M., Ishibashi, M., Nakane, A., Nakano, T. & Hirota, Y. Differences in COVID-19 vaccine acceptance, hesitancy, and confidence between healthcare workers and the general population in Japan. Vaccines 9 , 1389 (2021).
Holeva, V., Parlapani, E., Nikopoulou, V. A., Nouskas, I. & Diakogiannis, I. COVID-19 vaccine hesitancy in a sample of Greek adults. Psychol. Health Med. 27 , 113–119 (2022).
Hong, J. et al. Knowledge about, attitude and acceptance towards, and predictors of intention to receive the COVID-19 vaccine among cancer patients in eastern China: a cross-sectional survey. J. Integr. Med. 20 , 34–44 (2021).
Hossain, M. B. et al. COVID-19 vaccine hesitancy among the adult population in Bangladesh: a nationwide cross-sectional survey. PLoS ONE 16 , e0260821 (2021).
Hossain, M. B. et al. Health belief model, theory of planned behavior, or psychological antecedents: what predicts covid-19 vaccine hesitancy better among the Bangladeshi adults? Front. Public Health 9 , 711066 (2021).
Huang, H. et al. COVID-19 vaccine uptake, acceptance, and hesitancy among persons with mental disorders during the second stage of China’s nationwide vaccine rollout. Front. Med. 8 , 761601 (2021).
Hussain, S. et al. Perceptions regarding COVID-19 vaccination among a representative Pakistani population coming to tertiary care cardiac hospital. Cureus 13 , e18654 (2021).
Hwang, S. E., Kim, W. H. & Heo, J. Socio-demographic, psychological, and experiential predictors of COVID-19 vaccine hesitancy in South Korea, October–December 2020. Hum. Vaccin. Immunother. 18 , 1–8 (2021).
Hyland, P. et al. Detecting and describing stability and change in COVID-19 vaccine receptibility in the United Kingdom and Ireland. PLoS ONE 16 , e0258871 (2021).
Issanov, A., Akhmetzhanova, Z., Riethmacher, D. & Aljofan, M. Knowledge, attitude, and practice toward COVID-19 vaccination in Kazakhstan: a cross-sectional study. Hum. Vaccin. Immunother. 17 , 3394–3400 (2021).
Jain, J. et al. COVID-19 vaccine hesitancy among medical students in India. Epidemiol. Infect. 149 , e132 (2021).
Kanyike, A. M. et al. Acceptance of the coronavirus disease-2019 vaccine among medical students in Uganda. Trop. Med. Health 49 , 37 (2021).
Kateeb, E. et al. Predictors of willingness to receive COVID-19 vaccine: cross-sectional study of Palestinian dental students. Vaccines 9 , 954 (2021).
Khairat, S., Zou, B. & Adler-Milstein, J. Factors and reasons associated with low COVID-19 vaccine uptake among highly hesitant communities in the US. Am. J. Infect. Control 50 , 262–267 (2022).
Khan, M. S. R., Watanapongvanich, S. & Kadoya, Y. COVID-19 vaccine hesitancy among the younger generation in Japan. Int. J. Environ. Res. Public Health 18 , 11702 (2021).
Khodadadi, A. B., Hansen, B., Kim, Y. I. & Scarinci, I. C. Latinx immigrant mothers’ perceived self-efficacy and intentions regarding human papillomavirus vaccination of their daughters. Womens Health Issues 32 , 293–300 (2022).
King, W. C., Rubinstein, M., Reinhart, A. & Mejia, R. Time trends, factors associated with, and reasons for COVID-19 vaccine hesitancy: a massive online survey of US adults from January–May 2021. PLoS ONE 16 , e0260731 (2021).
King, W. C., Rubinstein, M., Reinhart, A. & Mejia, R. COVID-19 vaccine hesitancy January–May 2021 among 18–64 year old US adults by employment and occupation. Prev. Med. Rep. 24 , 101569 (2021).
Ko, T., Dendle, C., Woolley, I., Morand, E. & Antony, A. SARS-COV-2 vaccine acceptance in patients with rheumatic diseases: a cross-sectional study. Hum. Vaccin. Immunother. 17 , 4048–4056 (2021).
Kollamparambil, U., Oyenubi, A. & Nwosu, C. COVID19 vaccine intentions in South Africa: health communication strategy to address vaccine hesitancy. BMC Public Health 21 , 2113 (2021).
Kozak, A. & Nienhaus, A. COVID-19 vaccination: status and willingness to be vaccinated among employees in health and welfare care in Germany. Int. J. Environ. Res. Public Health 18 , 6688 (2021).
Krishnamurthy, K. et al. COVID-19 vaccine intent among health care professionals of Queen Elizabeth Hospital, Barbados. J. Multidiscip. Healthc. 14 , 3309–3319 (2021).
Ku, L. The association of social factors and health insurance coverage with COVID-19 vaccinations and hesitancy, July 2021. J. Gen. Intern. Med. 37 , 409–414 (2022).
Kuhn, R. et al. COVID-19 vaccine access and attitudes among people experiencing homelessness from pilot mobile phone survey in Los Angeles, CA. PLoS ONE 16 , e0255246 (2021).
Kumar, R., Alabdulla, M., Elhassan, N. M. & Reagu, S. M. Qatar healthcare workers’ COVID-19 vaccine hesitancy and attitudes: a national cross-sectional survey. Front. Public Health 9 , 727748 (2021).
Lang, R. et al. COVID-19 vaccine attitudes and beliefs: a Canadian national cross-sectional survey and cluster analysis. JMIR Public Health Surveill. 7 , e30424 (2021).
Lee, M. & You, M. Direct and indirect associations of media use with COVID-19 vaccine hesitancy in South Korea: cross-sectional web-based survey. J. Med. Internet Res. 24 , e32329 (2022).
Li, M. et al. Hesitancy toward COVID-19 vaccines among medical students in Southwest China: a cross-sectional study. Hum. Vaccin. Immunother. 17 , 4021–4027 (2021).
Liddell, B. J. et al. Factors associated with COVID-19 vaccine hesitancy amongst refugees in Australia. Eur. J. Psychotraumatol. 12 , 1997173 (2021).
Lim, L. J., Lim, A. J. W., Fong, K. K. & Lee, C. G. Sentiments regarding COVID-19 vaccination among graduate students in Singapore. Vaccines 9 , 1141 (2021).
Liu, R. & Li, G. M. Hesitancy in the time of coronavirus: temporal, spatial, and sociodemographic variations in COVID-19 vaccine hesitancy. SSM Popul. Health 15 , 100896 (2021).
Longchamps, C. et al. COVID-19 vaccine hesitancy among persons living in homeless shelters in France. Vaccine 39 , 3315–3318 (2021).
Luk, T. T. et al. Prevalence and determinants of SARS-CoV-2 vaccine hesitancy in Hong Kong: a population-based survey. Vaccine 39 , 3602–3607 (2021).
Luo, H. et al. Willingness to get a COVID-19 vaccine and reasons for hesitancy among medicare beneficiaries: results from a national survey. J. Public Health Manage. Pract. 28 , 70–76 (2022).
Magon, A. et al. The effect of health literacy on vaccine hesitancy among Italian anticoagulated population during COVID-19 pandemic: the moderating role of health engagement. Hum. Vaccin. Immunother. 17 , 5007–5012 (2021).
Martinelli, M. & Veltri, G. A. Do cognitive styles affect vaccine hesitancy? A dual-process cognitive framework for vaccine hesitancy and the role of risk perceptions. Soc. Sci. Med . 289 , 114403 (2021).
McCabe, S. D. et al. Unraveling Attributes of COVID-19 vaccine hesitancy and uptake in the U.S.: a large nationwide study. Preprint at medRxiv https://doi.org/10.1101/2021.04.05.21254918 (2021).
McCarthy, M., Murphy, K., Sargeant, E. & Williamson, H. Examining the relationship between conspiracy theories and covid-19 vaccine hesitancy: a mediating role for perceived health threats, trust, and anomie? Anal. Soc. Issues Public Policy 22 , 106–129 (2022).
McElfish, P. A. et al. Sociodemographic determinants of COVID-19 vaccine hesitancy, fear of infection, and protection self-efficacy. J. Prim. Care Community Health 12 , 21501327211040746 (2021).
Milošević Đorđević, J., Mari, S., Vdović, M. & Milošević, A. Links between conspiracy beliefs, vaccine knowledge, and trust: anti-vaccine behavior of Serbian adults. Soc. Sci. Med . 277 , 113930 (2021).
Mohamad, O. et al. Factors associated with the intention of Syrian adult population to accept COVID19 vaccination: a cross-sectional study. BMC Public Health 21 , 1310 (2021).
Mohan, S., Reagu, S., Lindow, S. & Alabdulla, M. COVID-19 vaccine hesitancy in perinatal women: a cross sectional survey. J. Perinat. Med. 49 , 678–685 (2021).
Mohanan, L., Klugar, M., Pokorna, A. & Riad, A. COVID-19 vaccine booster hesitancy (VBH) of healthcare workers in Czechia: national cross-sectional study. Vaccines 9 , 1437 (2021).
Momplaisir, F. M. et al. Racial/ethnic differences in COVID-19 vaccine hesitancy among health care workers in 2 large academic hospitals. JAMA Netw. Open 4 , e2121931 (2021).
Monami, M. et al. COVID-19 vaccine hesitancy and early adverse events reported in a cohort of 7,881 Italian physicians. Ann. Ig. 34 , 344–357 (2022).
Moore, D. et al. Low COVID-19 vaccine hesitancy in Brazil. Vaccine 39 , 6262–6268 (2021).
Morris, J. L., Baniak, L. M., Luyster, F. S. & Dunbar-Jacob, J. Covid-19 vaccine confidence and hesitancy in nursing students and faculty at a large academic medical center. Nurs. Outlook 70 , 347–354 (2022).
Muhajarine, N. et al. COVID-19 vaccine hesitancy and refusal and associated factors in an adult population in Saskatchewan, Canada: evidence from predictive modelling. PLoS ONE 16 , e0259513 (2021).
Myers, A., Ipsen, C. & Lissau, A. COVID-19 vaccination hesitancy among Americans with disabilities aged 18-65: An exploratory analysis. Disabil. Health J. 15 , 101223 (2022).
Nowak, S. A., Gidengil, C. A., Parker, A. M. & Matthews, L. J. Association among trust in health care providers, friends, and family, and vaccine hesitancy. Vaccine 39 , 5737–5740 (2021).
Okubo, R., Yoshioka, T., Ohfuji, S., Matsuo, T. & Tabuchi, T. COVID-19 vaccine hesitancy and its associated factors in Japan. Vaccines 9 , 662 (2021).
Oliveira, B. et al. Prevalence and factors associated with covid-19 vaccine hesitancy in Maranhão, Brazil. Rev. Saude Publica 55 , 12 (2021).
Omar, D. I. & Hani, B. M. Attitudes and intentions towards COVID-19 vaccines and associated factors among Egyptian adults. J. Infect. Public Health 14 , 1481–1488 (2021).
Orangi, S. et al. Assessing the level and determinants of COVID-19 vaccine confidence in Kenya. Vaccines 9 , 936 (2021).
Pal, S. et al. COVID-19 vaccine hesitancy and attitude toward booster doses among US healthcare workers. Vaccines 9 , 1358 (2021).
Park, H. K., Ham, J. H., Jang, D. H., Lee, J. Y. & Jang, W. M. Political ideologies, government trust, and COVID-19 vaccine hesitancy in South Korea: a cross-sectional survey. Int. J. Environ. Res. Public Health 18 , 10655 (2021).
Piltch-Loeb, R. et al. COVID-19 vaccine concerns about safety, effectiveness, and policies in the United States, Canada, Sweden, and Italy among unvaccinated individuals. Vaccines 9 , 1138 (2021).
Purnell, M. et al. Exploring COVID-19 vaccine hesitancy at a rural historically black college and university. J. Am. Pharm. Assoc. 62 , 340–344 (2021).
Purvis, R. S. et al. Trusted sources of COVID-19 vaccine information among hesitant adopters in the United States. Vaccines 9 , 1418 (2021).
Qunaibi, E. A., Helmy, M., Basheti, I. & Sultan, I. A high rate of COVID-19 vaccine hesitancy in a large-scale survey on Arabs. eLife 10 , e68038 (2021).
Riad, A. et al. Prevalence and drivers of COVID-19 vaccine hesitancy among Czech university students: national cross-sectional study. Vaccines 9 , 948 (2021).
Lavoie, K. et al. Understanding national trends in COVID-19 vaccine hesitancy in Canada - April 2020 to March 2021. BMJ Open 12 , e059411 (2022).
Robertson, E. et al. Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain Behav. Immun. 94 , 41–50 (2021).
Rodriguez, R. M. et al. The rapid evaluation of COVID-19 vaccination in emergency departments for underserved patients study. Ann. Emerg. Med. 78 , 502–510 (2021).
Saluja, S. et al. Disparities in COVID-19 vaccine hesitancy among Los Angeles County adults after vaccine authorization. Prev. Med. Rep. 24 , 101544 (2021).
Shallal, A. et al. Evaluation of COVID-19 vaccine attitudes among Arab American healthcare professionals living in the United States. Vaccines 9 , 942 (2021).
Sirikalyanpaiboon, M. et al. COVID-19 vaccine acceptance, hesitancy, and determinants among physicians in a university-based teaching hospital in Thailand. BMC Infect. Dis. 21 , 1174 (2021).
Solak, Ç., Peker-Dural, H., Karlıdağ, S. & Peker, M. Linking the behavioral immune system to COVID-19 vaccination intention: the mediating role of the need for cognitive closure and vaccine hesitancy. Pers. Individ. Dif. 185 , 111245 (2022).
Spinewine, A. et al. Attitudes towards COVID-19 vaccination among hospital staff-understanding what matters to hesitant people. Vaccines 9 , 469 (2021).
Stojanovic, J. et al. Global trends and correlates of COVID-19 vaccination hesitancy: findings from the iCARE study. Vaccines 9 , 661 (2021).
Strathdee, S. A. et al. Correlates of coronavirus disease 2019 (COVID-19) vaccine hesitancy among people who inject drugs in the San Diego-Tijuana border region. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciab975 (2021).
Szilagyi, P. G. et al. The role of trust in the likelihood of receiving a COVID-19 vaccine: results from a national survey. Prev. Med. 153 , 106727 (2021).
Szilagyi, P. G. et al. Likelihood of COVID-19 vaccination by subgroups across the US: post-election trends and disparities. Hum. Vaccin. Immunother. 17 , 3262–3267 (2021).
Tahir, A. I. et al. COVID-19 vaccine acceptance, hesitancy and refusal among Iraqi Kurdish population. Int. J. Health Sci. 16 , 10–16 (2022).
Teasdale, C. A. et al. Parental plans to vaccinate children for COVID-19 in New York city. Vaccine 39 , 5082–5086 (2021).
Thanapluetiwong, S., Chansirikarnjana, S., Sriwannopas, O., Assavapokee, T. & Ittasakul, P. Factors associated with COVID-19 vaccine hesitancy in Thai seniors. Patient Prefer. Adherence 15 , 2389–2403 (2021).
Tsai, R. et al. COVID-19 vaccine hesitancy and acceptance among individuals with cancer, autoimmune diseases, or other serious comorbid conditions: cross-sectional, internet-based survey. JMIR Public Health Surveill. 8 , e29872 (2022).
Uzochukwu, I. C. et al. COVID-19 vaccine hesitancy among staff and students in a Nigerian tertiary educational institution. Ther. Adv. Infect. Dis. 8 , 20499361211054923 (2021).
Vieira Rezende, R. P. et al. Characteristics associated with COVID-19 vaccine hesitancy: a nationwide survey of 1000 patients with immune-mediated inflammatory diseases. Vaccine 39 , 6454–6459 (2021).
Wang, S. X., Bell-Rogers, N., Dillard, D. & Harrington, M. A. COVID-19 vaccine hesitancy in Delaware’s underserved communities. Dela. J. Public Health 7 , 168–175 (2021).
Wang, X., Lin, L., Xu, J., Wang, W. & Zhou, X. Expectant parents’ vaccine decisions influenced by the 2018 Chinese vaccine crisis: a cross-sectional study. Prev. Med. 145 , 106423 (2021).
Waring, M. E. et al. Factors associated with mothers’ hesitancy to receive a COVID-19 vaccine. J. Behav. Med . https://doi.org/10.1007/s10865-021-00268-0 (2022).
Waters, A. R. et al. COVID-19 vaccine hesitancy among adolescent and young adult cancer survivors. JNCI Cancer Spectr. 5 , pkab049 (2021).
West, H. et al. COVID-19 vaccine hesitancy among temporary foreign workers from Bangladesh. Health Syst. Reform 7 , e1991550 (2021).
Willis, D. E. et al. COVID-19 vaccine hesitancy: race/ethnicity, trust, and fear. Clin. Transl. Sci. 14 , 2200–2207 (2021).
Willis, D. E., Presley, J., Williams, M., Zaller, N. & McElfish, P. A. COVID-19 vaccine hesitancy among youth. Hum. Vaccin. Immunother. 17 , 5013–5015 (2021).
Wiysonge, C. S. et al. COVID-19 vaccine acceptance and hesitancy among healthcare workers in South Africa. Expert Rev. Vaccines 21 , 549–559 (2022).
Wu, H., Ward, M., Brown, A., Blackwell, E. & Umer, A. COVID-19 vaccine intent in Appalachian patients with multiple sclerosis. Mult. Scler. Relat. Disord. 57 , 103450 (2021).
Wu, J. et al. COVID-19 vaccine hesitancy among Chinese population: a large-scale national study. Front. Immunol. 12 , 781161 (2021).
Xu, Y. et al. A cross-sectional survey on covid-19 vaccine hesitancy among parents from Shandong vs. Zhejiang. Front. Public Health 9 , 779720 (2021).
Zhang, M. X., Lin, X. Q., Chen, Y., Tung, T. H. & Zhu, J. S. Determinants of parental hesitancy to vaccinate their children against COVID-19 in China. Expert Rev. Vaccines 20 , 1339–1349 (2021).
Zhang, P. et al. Who is more likely to hesitate to accept COVID-19 vaccine: a cross-sectional survey in China. Expert Rev. Vaccines 21 , 397–406 (2022).
Zhao, Y. M. et al. Public willingness and determinants of COVID-19 vaccination at the initial stage of mass vaccination in China. Vaccines 9 , 1172 (2021).
Opel, D. J. et al. Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine 29 , 6598–6605 (2011).
Abd Halim, H., Abdul-Razak, S., Md Yasin, M. & Isa, M. R. Validation study of the Parent Attitudes About Childhood Vaccines (PACV) questionnaire: the Malay version. Hum. Vaccin. Immunother. 16 , 1040–1049 (2020).
Al-Regaiey, K. A. et al. Influence of social media on parents’ attitudes towards vaccine administration. Hum. Vaccin. Immunother. 18 , 1872340 (2021).
Alsuwaidi, A. R. et al. Vaccine hesitancy and its determinants among Arab parents: a cross-sectional survey in the United Arab Emirates. Hum. Vaccin. Immunother. 16 , 3163–3169 (2020).
Bagateli, L. E. et al. COVID-19 vaccine hesitancy among parents of children and adolescents living in Brazil. Vaccines 9 , 1115 (2021).
Beck, A., Bianchi, A. & Showalter, D. Evidence-based practice model to increase human papillomavirus vaccine uptake: a stepwise approach. Nurs. Womens Health 25 , 430–436 (2021).
Bianco, A., Mascaro, V., Zucco, R. & Pavia, M. Parent perspectives on childhood vaccination: how to deal with vaccine hesitancy and refusal? Vaccine 37 , 984–990 (2019).
Bonsu, N. E. M. et al. Understanding vaccine hesitancy among parents of children with autism spectrum disorder and parents of children with non-autism developmental delays. J. Child Neurol. 36 , 911–918 (2021).
Bulun, M. A. & Acuner, D. Turkish adaptation and reliability and validity study of parent attitudes about childhood vaccines survey. J. Pediatr. Res. 7 , 323–330 (2020).
Chang, J. & Kochel, R. Vaccine hesitancy and attributions for autism among racially and ethnically diverse groups of parents of children with autism spectrum disorder: a pilot study. Autism Res. 13 , 1790–1796 (2020).
Chung-Delgado, K., Valdivia Venero, J. E. & Vu, T. M. Vaccine hesitancy: characteristics of the refusal of childhood vaccination in a Peruvian population. Cureus 13 , e14105 (2021).
Clayton, K., Finley, C., Flynn, D. J., Graves, M. & Nyhan, B. Evaluating the effects of vaccine messaging on immunization intentions and behavior: evidence from two randomized controlled trials in Vermont. Vaccine 39 , 5909–5917 (2021).
Cole, J., Berman, S., Gardner, J., McGuire, K. & Chen, A. M. H. Implementation of a motivational interviewing-based decision tool to improve childhood vaccination rates: pilot study protocol. Res. Social Adm. Pharm. 17 , 619–624 (2020).
Cunningham, R. M., Guffey, D., Minard, C. G., Opel, D. J. & Boom, J. A. The effect of screening for vaccine hesitancy on the subsequent development of hesitancy: a randomized controlled trial, Houston, TX. Hum. Vaccin. Immunother. 17 , 1994–2000 (2021).
Daley, M. F., Narwaney, K. J., Shoup, J. A., Wagner, N. M. & Glanz, J. M. Addressing parents’ vaccine concerns: a randomized trial of a social media intervention. Am. J. Prev. Med . 55 , 44–54 (2018).
Eby, A. Z. Impacting parental vaccine decision-making. Pediatr. Nurs. 43 , 22–29, 34 (2017).
Goin-Kochel, R. P. et al. Beliefs about causes of autism and vaccine hesitancy among parents of children with autism spectrum disorder. Vaccine 38 , 6327–6333 (2020).
Henrikson, N. B. et al. Longitudinal trends in vaccine hesitancy in a cohort of mothers surveyed in Washington State, 2013–2015. Public Health Rep. 132 , 451–454 (2017).
Henrikson, N. B. et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics 136 , 70–79 (2015).
Hofstetter, A. M. et al. Parental vaccine hesitancy and declination of influenza vaccination among hospitalized children. Hosp. Pediatr. 8 , 628–635 (2018).
Kalok, A. et al. Vaccine hesitancy towards childhood immunisation amongst urban pregnant mothers in Malaysia. Vaccine 38 , 2183–2189 (2020).
Langkamp, D. L., Dusseau, A. & Brown, M. F. Vaccine hesitancy and low immunization rates in children with Down Syndrome. J. Pediatr. 223 , 64–67 (2020).
Mical, R., Martin-Velez, J., Blackstone, T. & Derouin, A. Vaccine hesitancy in rural pediatric primary care. J. Pediatr. Health Care 35 , 16–22 (2020).
Mohd Azizi, F. S., Kew, Y. & Moy, F. M. Vaccine hesitancy among parents in a multi-ethnic country, Malaysia. Vaccine 35 , 2955–2961 (2017).
Napolitano, F., D’Alessandro, A. & Angelillo, I. F. Investigating Italian parents’ vaccine hesitancy: a cross-sectional survey. Hum. Vaccin. Immunother. 14 , 1558–1565 (2018).
Olarewaju, V. O. et al. Application of the Parent Attitudes about Childhood Vaccines (PACV) survey in three national languages in Switzerland: exploratory factor analysis and Mokken scale analysis. Hum. Vaccin. Immunother. 17 , 2652–2660 (2021).
Opel, D. J. et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics 132 , 1037–1046 (2013).
Opel, D. J. et al. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: a validation study. JAMA Pediatr. 167 , 1065–1071 (2013).
Opel, D. J. et al. Impact of childhood vaccine discussion format over time on immunization status. Acad. Pediatr. 18 , 430–436 (2018).
Orr, C. & Beck, A. F. Measuring vaccine hesitancy in a minority community. Clin. Pediatr. 56 , 784–788 (2017).
Reuben, R., Aitken, D., Freedman, J. L. & Einstein, G. Mistrust of the medical profession and higher disgust sensitivity predict parental vaccine hesitancy. PLoS ONE 15 , e0237755 (2020).
Sabahelzain, M. M., van den Borne, B., Moukhyer, M. & Bosma, H. Determinants of measles vaccine hesitancy among Sudanese parents in Khartoum State, Sudan: a cross-sectional study. Vaccines 10 , 6 (2022).
Sahni, L. C. et al. Vaccine hesitancy and illness perceptions: comparing parents of children with autism spectrum disorder to other parent groups. Child. Health Care 35 , 385–402 (2020).
Sankaranarayanan, S., Jayaraman, A. & Gopichandran, V. Assessment of vaccine hesitancy among parents of children between 1 and 5 years of age at a tertiary care hospital in Chennai. Indian J. Community Med. 44 , 394–396 (2019).
Strelitz, B. et al. Parental vaccine hesitancy and acceptance of seasonal influenza vaccine in the pediatric emergency department. Vaccine 33 , 1802–1807 (2015).
Wagner, A. L. et al. Vaccine hesitancy and concerns about vaccine safety and effectiveness in Shanghai, China. Am. J. Prev. Med . 60 , S77–S86 (2020).
Williams, J. T. B., Rice, J. D. & O’Leary, S. T. Associations between religion, religiosity, and parental vaccine hesitancy. Vaccine X 9 , 100121 (2021).
Williams, S. E. et al. Screening tool predicts future underimmunization among a pediatric practice in Tennessee. Clin. Pediatr. 55 , 537–542 (2016).
Williams, S. E. et al. A randomized trial to increase acceptance of childhood vaccines by vaccine-hesitant parents: a pilot study. Acad. Pediatr. 13 , 475–480 (2013).
Yufika, A. et al. Parents’ hesitancy towards vaccination in Indonesia: a cross-sectional study in Indonesia. Vaccine 38 , 2592–2599 (2020).
Akhmetzhanova, Z., Sazonov, V., Riethmacher, D. & Aljofan, M. Vaccine adherence: the rate of hesitancy toward childhood immunization in Kazakhstan. Expert Rev. Vaccines 19 , 579–584 (2020).
Cunningham, R. M. et al. Development of a Spanish version of the parent attitudes about childhood vaccines survey. Hum. Vaccin. Immunother. 15 , 1106–1110 (2019).
Cunningham, R. M. et al. Prevalence of vaccine hesitancy among expectant mothers in Houston, Texas. Acad. Pediatr. 18 , 154–160 (2018).
Della Polla, G., Pelullo, C. P., Napolitano, F. & Angelillo, I. F. HPV vaccine hesitancy among parents in Italy: a cross-sectional study. Hum. Vaccin. Immunother. 16 , 2744–2751 (2020).
Dubé, E. et al. Overview of knowledge, attitudes, beliefs, vaccine hesitancy and vaccine acceptance among mothers of infants in Quebec, Canada. Hum. Vaccin. Immunother. 15 , 113–120 (2019).
Marshall, S., Moore, A. C., Sahm, L. J. & Fleming, A. Parent attitudes about childhood vaccines: point prevalence survey of vaccine hesitancy in an Irish population. Pharmacy 9 , 188 (2021).
Moyer-Gusé, E., Robinson, M. J. & McKnight, J. The role of humor in messaging about the MMR vaccine. J. Health Commun. 23 , 514–522 (2018).
Olarewaju, V. O. et al. The Youth Attitudes about Vaccines (YAV-5) scale: adapting the parent attitudes about childhood vaccines short scale for use with youth in German, French, and Italian in Switzerland, exploratory factor analysis and mokken scaling analysis. Hum. Vaccin. Immunother. 17 , 5183–5190 (2021).
Roberts, J. R. et al. Vaccine hesitancy among parents of adolescents and its association with vaccine uptake. Vaccine 33 , 1748–1755 (2015).
Ruggiero, K. M. et al. Parents’ intentions to vaccinate their children against COVID-19. J. Pediatr. Health Care 35 , 509–517 (2021).
Voo, J. Y. H. et al. Vaccine knowledge, awareness and hesitancy: a cross sectional survey among parents residing at Sandakan District, Sabah. Vaccines 9 , 1348 (2021).
Wallace, A. S. et al. Development of a valid and reliable scale to assess parents’ beliefs and attitudes about childhood vaccines and their association with vaccination uptake and delay in Ghana. Vaccine 37 , 848–856 (2019).
Wang, Y. & Zhang, X. Influence of parental psychological flexibility on pediatric COVID-19 vaccine hesitancy: mediating role of self-efficacy and coping style. Front. Psychol. 12 , 783401 (2021).
Whelan, S. O., Moriarty, F., Lawlor, L., Gorman, K. M. & Beamish, J. Vaccine hesitancy and reported non-vaccination in an Irish pediatric outpatient population. Eur. J. Pediatr. 180 , 2839–2847 (2021).
Williams, J. T. B. & O’Leary, S. T. Denver religious leaders’ vaccine attitudes, practices, and congregational experiences. J. Relig. Health 58 , 1356–1367 (2019).
Yörük, S. & Güler, D. Factors associated with pediatric vaccine hesitancy of parents: a cross-sectional study in Turkey. Hum. Vaccin. Immunother. 17 , 4505–4511 (2021).
Zhang, H. et al. Vaccine hesitancy among parents and its influencing factors: a cross-sectional study in Guangzhou, China. Hum. Vaccin. Immunother. 17 , 5153–5161 (2021).
Cataldi, J. R., Dempsey, A. F., Allison, M. A. & O’Leary, S. T. Impact of publicly available vaccination rates on parental school and child care choice. Vaccine 36 , 4525–4531 (2018).
Cataldi, J. R., Dempsey, A. F. & O’Leary, S. T. Measles, the media, and MMR: impact of the 2014–15 measles outbreak. Vaccine 34 , 6375–6380 (2016).
Cataldi, J. R. et al. Addressing personal parental values in decisions about childhood vaccination: measure development. Vaccine 37 , 5688–5697 (2019).
McDonald, C. et al. a consent support resource with benefits and harms of vaccination does not increase hesitancy in parents–an acceptability study. Vaccines 8 , 500 (2020).
Opel, D. J. et al. Previsit screening for parental vaccine hesitancy: a cluster randomized trial. Pediatrics 144 , e20190802 (2019).
Williams, J. T. B. et al. Adapting and piloting a vaccine hesitancy questionnaire in rural Guatemala. Vaccine 39 , 180–184 (2021).
Akbas Gunes, N. Parents’ perspectives about vaccine hesitancies and vaccine rejection, in the west of Turkey. J. Pediatr. Nurs. 53 , e186–e194 (2020).
Aldakhil, H., Albedah, N., Alturaiki, N., Alajlan, R. & Abusalih, H. Vaccine hesitancy towards childhood immunizations as a predictor of mothers’ intention to vaccinate their children against COVID-19 in Saudi Arabia. J. Infect. Public Health 14 , 1497–1504 (2021).
AlGoraini, Y. M. et al. Confidence toward vaccination as reported by parents of children admitted to a tertiary care hospital in Riyadh, Saudi Arabia: a cross sectional study. Vacunas 21 , 95–104 (2020).
Alsubaie, S. S. et al. Vaccine hesitancy among Saudi parents and its determinants. Result from the WHO SAGE working group on vaccine hesitancy survey tool. Saudi Med. J. 40 , 1242–1250 (2019).
Connors, J. T., Hodges, E. A., DʼAuria, J. & Windham, L. Implementing vaccine hesitancy screening for targeted education. J. Am. Assoc. Nurse Pract. 30 , 450–459 (2018).
Dasgupta, P., Bhattacherjee, S., Mukherjee, A. & Dasgupta, S. Vaccine hesitancy for childhood vaccinations in slum areas of Siliguri, India. Indian J. Public Health 62 , 253–258 (2018).
Johnson, K. D. et al. Combatting a “twin-demic”: a quantitative assessment of COVID-19 and influenza vaccine hesitancy in primary care patients. Health Promot. Perspect. 11 , 179–185 (2021).
Krishnamoorthy, Y. et al. Factors related to vaccine hesitancy during the implementation of Measles-Rubella campaign 2017 in rural Puducherry–a mixed-method study. J. Fam. Med. Prim. Care 8 , 3962–3970 (2019).
Masters, N. B., Tefera, Y. A., Wagner, A. L. & Boulton, M. L. Vaccine hesitancy among caregivers and association with childhood vaccination timeliness in Addis Ababa, Ethiopia. Hum. Vaccin. Immunother. 14 , 2340–2347 (2018).
Miko, D., Costache, C., Colosi, H. A., Neculicioiu, V. & Colosi, I. A. Qualitative assessment of vaccine hesitancy in Romania. Medicina 55 , 282 (2019).
Sharma, S., Akhtar, F., Singh, R. K. & Mehra, S. Understanding the three As (awareness, access, and acceptability) dimensions of vaccine hesitancy in Odisha, India. Clin. Epidemiol. Glob. Health 8 , 399–403 (2020).
Soysal, G., Durukan, E. & Akdur, R. The evaluation of vaccine hesitancy and refusal for childhood vaccines and the covid-19 vaccine in individuals aged between 18 and 25 years. Turkish J. Immunol. 9 , 120–127 (2021).
Khezong, A. et al. Association between adult vaccine hesitancy and parental acceptance of childhood COVID-19 vaccines: a web-based survey in a northwestern region in China. Vaccines 9 , 1088 (2021).
Akel, K. B., Masters, N. B., Shih, S. F., Lu, Y. & Wagner, A. L. Modification of a vaccine hesitancy scale for use in adult vaccinations in the United States and China. Hum. Vaccin. Immunother. 17 , 2639–2646 (2021).
Chen, M. et al. An online survey of the attitude and willingness of Chinese adults to receive COVID-19 vaccination. Hum. Vaccin. Immunother. 17 , 2279–2288 (2021).
Dolu, İ., Turhan, Z. & Yalnız Dilcen, H. COVID-19 vaccine acceptance is associated with vaccine hesitancy, perceived risk and previous vaccination experiences. Disaster Med. Public Health Prep . https://doi.org/10.1017/dmp.2021.370 (2021).
Domek, G. J. et al. Measuring vaccine hesitancy: field testing the WHO SAGE Working Group on Vaccine Hesitancy survey tool in Guatemala. Vaccine 36 , 5273–5281 (2018).
Gatwood, J. et al. Extent of and reasons for vaccine hesitancy in adults at high-risk for pneumococcal disease. Am. J. Health Promot . 35 , 908–916 (2021).
Han, Y. et al. Parental category B vaccine hesitancy and associated factors in China: an online cross-sectional survey. Expert Rev. Vaccines 21 , 145–153 (2021).
He, K., Mack, W. J., Neely, M., Lewis, L. & Anand, V. Parental perspectives on immunizations: impact of the COVID-19 pandemic on childhood vaccine hesitancy. J. Community Health 47 , 39–52 (2022).
Helmkamp, L. J. et al. A validated modification of the vaccine hesitancy scale for childhood, influenza and HPV vaccines. Vaccine 39 , 1831–1839 (2021).
Jones, D. L. et al. Severe acute respiratory syndrome coronavirus 2: vaccine hesitancy among underrepresented racial and ethnic groups with HIV in Miami, Florida. Open Forum Infect. Dis. 8 , ofab154 (2021).
Kasrine Al Halabi, C. et al. Attitudes of Lebanese adults regarding COVID-19 vaccination. BMC Public Health 21 , 998 (2021).
Kempe, A. et al. Parental hesitancy about routine childhood and influenza vaccinations: a national survey. Pediatrics 146 , e20193852 (2020).
Kim, H. W. et al. Perceptions of nurses on human papillomavirus vaccinations in the Republic of Korea. PLoS ONE 14 , e0211475 (2019).
Liu, P. L., Zhao, X. & Wan, B. COVID-19 information exposure and vaccine hesitancy: the influence of trust in government and vaccine confidence. Psychol. Health Med. https://doi.org/10.1080/13548506.2021.2014910 (2021).
Lu, F. & Sun, Y. COVID-19 vaccine hesitancy: the effects of combining direct and indirect online opinion cues on psychological reactance to health campaigns. Comput. Hum. Behav. 127 , 107057 (2022).
Lu, J. et al. Sensitivity to COVID-19 vaccine effectiveness and safety in Shanghai, China. Vaccines 9 , 472 (2021).
Lu, X. et al. Low willingness to vaccinate against herpes zoster in a Chinese metropolis. Hum. Vaccin. Immunother. 17 , 4163–4170 (2021).
Lu, X. et al. Gap between willingness and behavior in the vaccination against influenza, pneumonia, and herpes zoster among Chinese aged 50–69 years. Expert Rev. Vaccines 20 , 1147–1152 (2021).
Luyten, J., Bruyneel, L. & van Hoek, A. J. Assessing vaccine hesitancy in the UK population using a generalized vaccine hesitancy survey instrument. Vaccine 37 , 2494–2501 (2019).
Önal, Ö., Eroğlu, H. N., Evcil, F. Y., Kişioğlu, A. N. & Uskun, E. Validity and reliability of Turkish version of the Vaccine Hesitancy Scale. Turk. Arch. Pediatr. 56 , 230–235 (2021).
Ren, J. et al. The demographics of vaccine hesitancy in Shanghai, China. PLoS ONE 13 , e0209117 (2018).
Sabahelzain, M. M. et al. Psychometric properties of the adapted measles vaccine hesitancy scale in Sudan. PLoS ONE 15 , e0237171 (2020).
Sadaqat, W. et al. Determination of COVID-19 vaccine hesitancy among university students. Cureus 13 , e17283 (2021).
Shapiro, G. K. et al. The vaccine hesitancy scale: psychometric properties and validation. Vaccine 36 , 660–667 (2018).
Shen, X. et al. Assessing the COVID-19 vaccine hesitancy in the Chinese adults using a generalized vaccine hesitancy survey instrument. Hum. Vaccin. Immunother. 17 , 4005–4012 (2021).
Shih, S. F. et al. Vaccine hesitancy and rejection of a vaccine for the novel coronavirus in the United States. Front. Immunol. 12 , 558270 (2021).
Szilagyi, P. G. et al. Prevalence and characteristics of HPV vaccine hesitancy among parents of adolescents across the US. Vaccine 38 , 6027–6037 (2020).
Temsah, M. H. et al. Parental attitudes and hesitancy about COVID-19 vs. routine childhood vaccinations: a national survey. Front. Public Health 9 , 752323 (2021).
Thaker, J. The persistence of vaccine hesitancy: COVID-19 vaccination intention in New Zealand. J. Health Commun. 26 , 104–111 (2021).
Turhan, Z., Dilcen, H. Y. & Dolu, İ. The mediating role of health literacy on the relationship between health care system distrust and vaccine hesitancy during COVID-19 pandemic. Curr. Psychol . https://doi.org/10.1007/s12144-021-02105-8 (2021).
Vallis, M. & Glazer, S. Protecting individuals living with overweight and obesity: attitudes and concerns toward COVID-19 vaccination in Canada. Obesity 29 , 1128–1137 (2021).
Wagner, A. L. et al. Comparisons of vaccine hesitancy across five low- and middle-income countries. Vaccines 7 , 155 (2019).
Wagner, A. L., Shotwell, A. R., Boulton, M. L., Carlson, B. F. & Mathew, J. L. Demographics of vaccine hesitancy in Chandigarh, India. Front. Med. 7 , 585579 (2020).
Wang, Q. et al. Delays in routine childhood vaccinations and their relationship with parental vaccine hesitancy: a cross-sectional study in Wuxi, China. Expert Rev. Vaccines 21 , 135–143 (2021).
Wang, Q. et al. Vaccine hHesitancy: COVID-19 and influenza vaccine willingness among parents in Wuxi, China–a cross-sectional study. Vaccines 9 , 342 (2021).
Xu, Y. et al. COVID-19 vaccination attitudes with neuromyelitis optica spectrum disorders: vaccine hesitancy and coping style. Front. Neurol. 12 , 717111 (2021).
Yeşiltepe, A., Aslan, S. & Bulbuloglu, S. Investigation of perceived fear of COVID-19 and vaccine hesitancy in nursing students. Hum. Vaccin. Immunother. 17 , 5030–5037 (2021).
Duong, T. V. et al. Oxford COVID-19 vaccine hesitancy in school principals: impacts of gender, well-being, and coronavirus-related health literacy. Vaccines 9 , 958 (2021).
Guaracha-Basáñez, G. et al. COVID-19 vaccine hesitancy among Mexican outpatients with rheumatic diseases. Hum. Vaccin. Immunother. 17 , 5038–5047 (2021).
Joshi, A. et al. COVID-19 vaccine hesitancy in healthcare workers amidst the second wave of the pandemic in India: a single centre study. Cureus 13 , e17370 (2021).
Zhang, X., Guo, Y., Zhou, Q., Tan, Z. & Cao, J. The mediating roles of medical mistrust, knowledge, confidence and complacency of vaccines in the pathways from conspiracy beliefs to vaccine hesitancy. Vaccines 9 , 1342 (2021).
Pomares, T. D. et al. Association of cognitive biases with human papillomavirus vaccine hesitancy: a cross-sectional study. Hum. Vaccin. Immunother. 16 , 1018–1023 (2020).
Ashkenazi, S. et al. The relationship between parental source of information and knowledge about measles / measles vaccine and vaccine hesitancy. Vaccine 38 , 7292–7298 (2020).
Biswas, N., Mustapha, T., Khubchandani, J. & Price, J. H. The nature and extent of COVID-19 vaccination hesitancy in healthcare workers. J. Community Health 46 , 1244–1251 (2021).
Boattini, M. et al. Rubella serosurvey and factors related to vaccine hesitancy in childbearing women in Italy. Prev. Med. Rep. 15 , 100945 (2019).
Browne, S. K. et al. Coronavirus disease 2019 (COVID-19) vaccine hesitancy among physicians, physician assistants, nurse practitioners, and nurses in two academic hospitals in Philadelphia. Infect. Control Hosp. Epidemiol. https://doi.org/10.1017/ice.2021.410 (2021).
Dybsand, L. L., Hall, K. J. & Carson, P. J. Immunization attitudes, opinions, and knowledge of healthcare professional students at two Midwestern universities in the United States. BMC Med. Educ. 19 , 242 (2019).
Gao, X., Li, H., He, W. & Zeng, W. COVID-19 vaccine hesitancy among medical students: the next COVID-19 challenge in Wuhan, China. Disaster Med. Public Health Prep . https://doi.org/10.1017/dmp.2021.291 (2021).
Gerber, J. E. et al. Vaccinomics: a cross-sectional survey of public values. Hum. Vaccin. Immunother. 17 , 2999–3015 (2021).
Giambi, C. et al. Parental vaccine hesitancy in Italy – results from a national survey. Vaccine 36 , 779–787 (2018).
Guay, M., Gosselin, V., Petit, G., Baron, G. & Gagneur, A. Determinants of vaccine hesitancy in Quebec: a large population-based survey. Hum. Vaccin. Immunother. 15 , 2527–2533 (2019).
Harapan, H. et al. Vaccine hesitancy among communities in ten countries in Asia, Africa, and South America during the COVID-19 pandemic. Pathog. Glob. Health 116 , 236–243 (2022).
Hornsey, M. J., Lobera, J. & Díaz-Catalán, C. Vaccine hesitancy is strongly associated with distrust of conventional medicine, and only weakly associated with trust in alternative medicine. Soc. Sci. Med. 255 , 113019 (2020).
Hu, Y., Chen, Y., Liang, H. & Wang, Y. Reliability and validity of a survey to identify vaccine hesitancy among parents in Changxing county, Zhejiang province. Hum. Vaccin. Immunother. 15 , 1092–1099 (2019).
Johnson, D. K. et al. Combating vaccine hesitancy with vaccine-preventable disease familiarization: an interview and curriculum intervention for college students. Vaccines 7 , 39 (2019).
Kassianos, G. et al. Motors of influenza vaccination uptake and vaccination advocacy in healthcare workers: a comparative study in six European countries. Vaccine 36 , 6546–6552 (2018).
Klassen, A. C. et al. Formative research to address vaccine hesitancy in Tajikistan. Vaccine 39 , 1516–1527 (2021).
Liu, Y. et al. COVID-19 vaccination in people living with HIV (PLWH) in China: a cross sectional study of vaccine hesitancy, safety, and immunogenicity. Vaccines 9 , 1458 (2021).
Paoli, S. et al. Assessing vaccine hesitancy among healthcare workers: a cross-sectional study at an Italian paediatric hospital and the development of a healthcare worker’s vaccination compliance index. Vaccines 7 , 201 (2019).
Rahman, M., Hossain, A., Sufian, A. & Anwar, N. COVID-19 vaccine demand, hesitancy, and nationalism: a case of protection motivation behavior in Bangladesh. J. Infect. Dev. Ctries 15 , 1388–1395 (2021).
Raude, J. et al. Opening the ‘Vaccine Hesitancy’ black box: how trust in institutions affects French GPs’ vaccination practices. Expert Rev. Vaccines 15 , 937–948 (2016).
Schwarzinger, M., Watson, V., Arwidson, P., Alla, F. & Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health 6 , e210–e221 (2021).
Stoeckel, F., Carter, C., Lyons, B. A. & Reifler, J. Association of vaccine hesitancy and immunization coverage rates in the European Union. Vaccine 39 , 3935–3939 (2021).
Tomljenovic, M., Petrovic, G., Antoljak, N. & Hansen, L. Vaccination attitudes, beliefs and behaviours among primary health care workers in northern Croatia. Vaccine 39 , 738–745 (2021).
Verger, P. et al. Vaccine hesitancy among hospital staff physicians: a cross-sectional survey in France in 2019. Vaccine 39 , 4481–4488 (2021).
Verger, P. et al. Vaccine hesitancy among general practitioners and its determinants during controversies: a national cross-sectional survey in France. EBioMedicine 2 , 891–897 (2015).
Carranza, D., Dub, T. & Sivelä, J. Vaccine Hesitancy and Uptake. From Research and Practices to Implementation (Finnish Institute for Health and Welfare (THL), 2021); https://eu-jav.com/public_deliverable/deliverable-8-1-vaccine-hesitancy-and-uptake-from-research-and-practices-to-implementation/
Merriam-Webster Dictionary Hesitancy https://www.merriam-webster.com/dictionary/hesitancy (2021).
Merriam-Webster Dictionary Vaccine Hesitancy https://www.merriam-webster.com/dictionary/vaccine%20hesitancy (2021).
Siani, A. et al. Investigating the determinants of vaccine hesitancy within undergraduate students’ social sphere. Z. Gesundh. Wiss . https://doi.org/10.1007/s10389-021-01538-6 (2021).
Strong, H., Reiter-Purtill, J., Howarth, T., West-Smith, L. & Zeller, M. H. Early COVID-19 vaccine hesitancy characteristics in mothers following bariatric surgery. Obes. Surg. 32 , 852–860 (2022).
Lavail, K. H. & Kennedy, A. M. The role of attitudes about vaccine safety, efficacy, and value in explaining parents’ reported vaccination behavior. Health Educ. Behav. 40 , 544–551 (2013).
Frew, P. M. et al. Development of a measure to assess vaccine confidence among men who have sex with men. Expert Rev. Vaccines 17 , 1053–1061 (2018).
World Bank Country and Lending Groups (The World Bank, 2021); https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
Hong, Q. N. et al. Mixed Methods Appraisal Tool (MMAT), version 2018 (Canadian Intellectual Property Office, Industry Canada, 2018).
Hong, Q. N. et al. Reporting the Results of the MMAT (Version 2018) http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/140056890/Reporting%20the%20results%20of%20the%20MMAT.pdf (2020).
Download references
Acknowledgements
J.L.A.H. and M.E.J.L.H. received funding from The Netherlands Organisation for Health Research and Development (ZonMw project number 839190002). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank J. van Haren for her valuable contribution in sorting and organizing the data of this systematic review.
Author information
Authors and affiliations.
Radboud University Medical Center, Radboud Institute for Health Sciences, Primary and Community Care, Nijmegen, the Netherlands
Daphne Bussink-Voorend, Jeannine L. A. Hautvast & Olga Visser
Behavioural Science Institute, Radboud University, Nijmegen, the Netherlands
Lisa Vandeberg
Radboud University Medical Center, Radboud Institute for Health Sciences, IQ Healthcare, Nijmegen, the Netherlands
Marlies E. J. L. Hulscher
You can also search for this author in PubMed Google Scholar
Contributions
D.B.-V., J.L.A.H., O.V. and M.E.J.L.H. designed the project and analysed the data. D.B.-V., J.L.A.H., L.V., O.V. and M.E.J.L.H. interpreted the data. The manuscript, figures and tables were drafted by D.B.-V. and edited by J.L.A.H., L.V., O.V. and M.E.J.L.H.
Corresponding author
Correspondence to Daphne Bussink-Voorend .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Human Behaviour thanks Chuanxi Fu, Amalie Dyda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
Supplementary methods, PRISMA 2020 for abstracts checklist, PRISMA 2020 checklist.
Reporting Summary
Peer review file, supplementary table 1.
Overview and characteristics of included studies.
Supplementary Table 2
Overview of extracted excerpts.
Supplementary Table 3
Overview of vaccine hesitancy subpopulations.
Supplementary Table 4
Overview of studies describing a measurement of vaccine hesitancy.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Bussink-Voorend, D., Hautvast, J.L.A., Vandeberg, L. et al. A systematic literature review to clarify the concept of vaccine hesitancy. Nat Hum Behav 6 , 1634–1648 (2022). https://doi.org/10.1038/s41562-022-01431-6
Download citation
Received : 25 November 2021
Accepted : 13 July 2022
Published : 22 August 2022
Issue Date : December 2022
DOI : https://doi.org/10.1038/s41562-022-01431-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Factors associated with sars-cov-2 vaccine hesitancy after stroke: a cross-sectional study.
- Ying-Hai Zhu
- Xiu-Qing Yao
BMC Public Health (2024)
- Cameron O’Neill Byerley
Scientific Reports (2024)
Vaccine hesitancy and trust in sub-Saharan Africa
- Kerstin Unfried

Association of personality traits and socio-environmental factors with COVID-19 pandemic-related conspiratorial thinking in the D-A-CH region
- Jakob Weitzer
- Gerald Steiner
SN Social Sciences (2024)
Modelling the impact of opinion flexibility on the vaccination choices during epidemics
- Rossella Della Marca
- Marco Menale
Ricerche di Matematica (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Efficacy and safety of COVID-19 vaccines: a systematic review
Covid-19疫苗的有效性和安全性的系统评价, xiao-yan tu, zhang-wu liang, jiang-nan chen, jiao-jiao li, li-guo jiang, fu-qiang xing.
- Author information
- Article notes
- Copyright and License information
Prof. JIANG Yi. Email: [email protected]
Received 2021 Jan 25; Accepted 2021 Feb 19.
To evaluate systematically the efficacy and safety of COVID-19 vaccines.
PubMed, Embase, Cochrane Library, Clinicaltrial.gov, CNKI, Wanfang Data, China Biomedical Literature Service System, and China Clinical Trial Registry were searched for randomized controlled trials of COVID-19 vaccines published up to December 31, 2020. The Cochrane bias risk assessment tool was used to assess the quality of studies. A qualitative analysis was performed on the results of clinical trials.
Thirteen randomized, blinded, controlled trials, which involved the safety and efficacy of 11 COVID-19 vaccines, were included. In 10 studies, the 28-day seroconversion rate of subjects exceeded 80%. In two 10 000-scale clinical trials, the vaccines were effective in 95% and 70.4% of the subjects, respectively. The seroconversion rate was lower than 60% in only one study. In six studies, the proportion of subjects who had an adverse reaction within 28 days after vaccination was lower than 30%. This proportion was 30%-50% in two studies and > 50% in the other two studies. Most of the adverse reactions were mild to moderate and resolved within 24 hours after vaccination. The most common local adverse reaction was pain or tenderness at the injection site, and the most common systemic adverse reaction was fatigue, fever, or bodily pain. The immune response and incidence of adverse reactions to the vaccines were positively correlated with the dose given to the subjects. The immune response to the vaccines was worse in the elderly than in the younger population. In 6 studies that compared single-dose and double-dose vaccination, 4 studies showed that double-dose vaccination produced a stronger immune response than single-dose vaccination.
Conclusions
Most of the COVID-19 vaccines appear to be effective and safe. Double-dose vaccination is recommended. However, more research is needed to investigate the long-term efficacy and safety of the vaccines and the influence of dose, age, and production process on the protective efficacy.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Systematic review, Efficacy, Safety, Clinical trial
系统评价新型冠状病毒肺炎(COVID-19)疫苗的有效性和安全性。
通过计算机检索有关COVID-19疫苗的临床随机对照试验文献,对临床试验结果进行定性分析。检索时间为各数据库建库至2020年12月31日。所检索的数据库包括PubMed、Embase、Cochrane图书馆、Clinicaltrial.gov、中国知网、万方数据、中国生物医学文献服务系统和中国临床试验注册中心。使用Cochrane偏倚风险评估工具评估文献质量。
纳入了13项随机、盲法、对照试验,涉及11种COVID-19疫苗接种的安全性和有效性。在其中10项研究中,受试者的28 d血清转化率超过80%;2项万人规模的临床试验中,分别取得了95%和70.4%的有效率;1项研究的血清转化率低于60%。在对接种后28 d内不良反应发生率的分析显示,6项研究不良反应发生率低于30%,2项研究为30%~50%,2项研究高于50%。在13项研究中,疫苗接种不良反应事件绝大部分为轻度到中度,在接种后24 h内缓解;最常见的局部不良反应为注射部位疼痛或压痛,最常见的系统性不良反应为疲劳、发热或躯体痛。受试者对疫苗的免疫反应和不良反应发生率与接种剂量呈正相关。老年人对疫苗的免疫反应较年轻人差。6项研究比较了疫苗单剂量与双剂量接种的效应,其中4项研究显示双剂量接种比单剂量接种产生更强的免疫反应。
大部分COVID-19疫苗具有较好的有效性和安全性;推荐双剂量接种。然而COVID-19疫苗的长期有效性、安全性及剂量、年龄和工艺差异对保护效力的影响需要更多的研究证实。
Keywords: 新型冠状病毒肺炎, 严重急性呼吸综合征冠状病毒2, 疫苗, 系统评价, 有效性, 安全性, 临床试验
It has been more than a year since the outbreak of the novel coronavirus pneumonia (COVID-19). Although the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that caused COVID-19 in China was effectively controlled, the global epidemic has not stopped. According to data from the World Health Organization, as of 16:05 on February 15, 2021, Central European Time, the cumulative number of confirmed COVID-19 cases worldwide reached 108, 579, 352, and the cumulative deaths reached 2, 396, 408 [ 1 ] . The COVID-19 epidemic as a major global public health event has become the primary health threat for all mankind, and impacted the world's political, economic and cultural greatly [ 2 - 3 ] . SARS-CoV-2 is a β-coronavirus with RNA as genetic material, which enters cell through a spike protein combined with angiotensin converting enzyme 2 [ 4 - 5 ] . COVID-19 generally manifests as fever and dry cough, and injuries multiple organ, especially the lungs [ 2 , 5 - 6 ] . Wearing mask and maintaining social distancing have been confirmed as the most effective measures to stop the spread of the virus form China's experience of fighting the epidemic [ 3 , 7 - 9 ] , and isolation and symptomatic supportive treatment still dominate for COVID-19 patients [ 5 ] . However, the efficacy of antiviral drugs and traditional Chinese medicines needs more evidence [ 10 - 11 ] . Due to the low penetration rate of masks and the limitations of treatment options abroad [ 12 - 13 ] , more and more hopes are pinned on the development of a COVID-19 vaccine. According to different targets and technologies, vaccines can be divided into the following categories: inactivated vaccines, recombinant spike protein vaccines, viral vector vaccines, RNA vaccines, live attenuated vaccines and virus-like particle vaccines, etc [ 14 - 16 ] . Currently, hundreds of COVID-19 candidate vaccine projects have been registered in the US clinical trial database (clinicaltrials.gov) [ 15 , 17 ] . Results of phase 3 clinical trials of several vaccines are published [ 18 - 22 ] . As of January 1, 2021, China, Russia, the United States, Britain and other countries have approved their own mass vaccination plans for the population. This study evaluated the safety and effectiveness of the COVID-19 vaccine through systematic literature review and qualitative analysis for the published COVID-19 vaccine clinical trial results.
1. Information and methods
This systematic review was completed in accordance with the guidelines in the "Preferred Reporting Project for Systematic Evaluation and Meta-Analysis (PRISMA)" [ 23 - 24 ] .
1.1. Literature inclusion criteria
The literature inclusion criteria: (1) The healthy men or non-pregnant women aged 18 and above; (2) COVID-19 vaccination as the intervention measure; (3) The randomized, controlled, and blinded trials; (4) The clinical trial results indicators include at least one or more as following: local adverse reactions (pain, itching, redness, swelling and induration, etc.), systemic adverse reactions (fever, diarrhea, fatigue, nausea/vomiting, etc.), the last vaccine neutralizing antibody geometric mean titer (GMT), seroconversion rate and other laboratory test indicators measured by live virus neutralization test 14 days or 28 days after inoculation.
1.2. Literature exclusion criteria
Documents that meet one of the following conditions were excluded: (1) Medical news, popular science articles, non-medical papers, reviews, letters, comments, basic research, case reports, conference abstracts; (2) No full text or literature published in a third language other than Chinese and English; (3) One of overlapping two studies were excluded; (4) If the data of the literature included in the later published literature, The former was excluded.
1.3. Literature search
The English databases PubMed, Embase, Cochrane Library and clinicaltrials.gov databases were searched. The Chinese databases searched included CNKI, Wanfang Database, China Biomedical Literature Service System and China Clinical Trial Registration Center. In order to ensure the comprehensiveness of the search results, this system evaluation used Boolean logic to search by "subject words + free words". The main search terms include: COVID-19, 2019-nCoV, SARS-CoV-2, 2019 novel coronavirus, vaccines, vaccination, COVID-19 vaccines, mRNA-1273 vaccine, Ad5-nCoV vaccine, ChAdOx1 COVID-19 vaccine, BNT162 vaccine, controlled clinical trial, randomized controlled trials, controlled clinical trial, random, blind, placebo, trial, Meta, and etc. Chinese search terms include: 新型冠状病毒、新冠肺炎、新型冠状病毒肺炎、疫苗、试验、随机对照试验、随机对照研究、随机对照、随机、元分析、Meta、荟萃, etc.
1.4. Literature screening and data extraction
The literature screening and data extraction were done independently by two researchers. Differences in the summary of the results will be discussed and dealt with by the two researchers or the third researcher. All results obtained in the database were imported into Note Express (Wuhan University Library Edition) software, and duplicate documents were removed mechanically using the software's duplicate check function. The initial screening by reading the title and abstract, and the secondary screening by reading the full text were completed. The extracted data included: the first author, vaccine type, inoculation dose, interval between inoculations, number of subjects and baseline characteristics (race, sex ratio, age range or average age), research design, local and systemic adverse reactions, laboratory indicators, as well as funds, sponsors and registration number.
1.5. Methodological quality evaluation
Assess the risk of bias according to the Cochrane Systematic Review Manual [ 25 - 26 ] .
1.6. Statistical analysis
The main results of this systematic review included the safety and effectiveness of the vaccine. Indicators for evaluating safety included local adverse reactions (pain, itching, redness, induration, etc.) and systemic adverse reactions (cough, diarrhea, fatigue, fever, headache, nausea/vomiting, itching, muscle pain, joint pain/discomfort, anorexia, etc.). The immunogenicity indicators included GMT, seroconversion rate, and the response of IgG or other specific antibodies to the receptor binding domain.
2.1. Literature search results
There were 753 relevant articles published before December 31, 2020. After screening, 13 papers were included in the system evaluation [ 19 - 22 , 27 - 35 ] . The process of document screening was shown in Figure 1 .
A flow diagram of literature screening
2.2. Methodological quality evaluation
The 13 included studies all adopted a randomized control method [ 19 - 22 , 27 - 35 ] , with a double-blind method in 10 studies [ 21 - 22 , 27 - 32 , 34 - 35 ] , and a single-blind method in 2 studies [ 20 , 33 ] , and bothsingle-blind method and double-blind methodin one study [ 19 ] . All trials hid the allocation plan. Nine trials had incomplete data or selective reports [ 19 , 22 , 27 , 29 - 31 , 33 - 35 ] , of which 2 had more missing data in the preprint [ 22 , 29 ] , and the remaining 7 missed individual data [ 19 , 27 , 30 - 31 , 33 - 35 ] ; 9 trials had other types of bias [ 19 - 20 , 22 , 29 - 32 , 34 - 35 ] , for example, Keech et al. [ 30 ] did not perform virus neutralization test in the experimental design. In general, the included literature had a low risk of bias ( Figure 2 & Table 1 ).
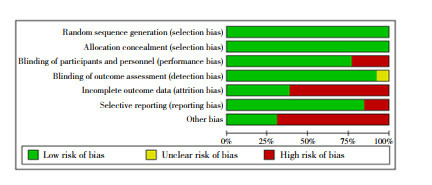
Risk assessment of literature bias
Methodological quality evaluation of included studies
2.3. The characteristics of the included studies
The 13 included studies were randomized, blinded, and controlled trials, involving 5 inactivated vaccines [ 21 - 22 , 27 - 29 , 34 ] , 2 recombinant spike protein vaccines [ 30 , 32 ] , 2 RNA vaccines [ 20 , 31 , 33 ] and 2 adenovirus vector vaccines [ 19 , 35 ] . Table 2 for details of vaccine characteristics and developer information). There were 6 studies comparing the effects of single-dose and double-dose vaccination [ 19 , 27 , 30 - 31 , 33 , 35 ] . Most of the 13 studies compared the difference of two doses of vaccine at intervals of 2, 3 or 4 weeks. Most studies also compared the difference between low, medium and high injection doses. Participants in all trials were adults, and 5 articles reported the results of vaccines in the elderly population [ 19 - 20 , 32 - 33 , 35 ] . The baseline characteristics of the participants were shown in Table 3 .
Experimental design and developers of the included studies
Baseline characteristics of the participants
2.4. Qualitative analysis
2.4.1. the effectiveness and safety of vaccines.
In 10 studies, the 28-day seroconversion rate of testee exceeded 80% [ 21 - 22 , 27 - 34 ] . The RNA vaccine (BNT162b2) reported by Polack achieved 95% efficiency [ 20 ] , the recombinant adenovirus vector vaccine (ChAdOx1 nCoV-19) reported by Voysey achieved an effective rate of 70.4% [ 19 ] , but Zhu reported that the 28-day seroconversion rate of the adenovirus recombinant vector vaccine in testee was less than 60% [ 35 ] .
In 6 studies, the incidence of adverse reactions in volunteers within 28 days for vaccination was less than 30% [ 20 - 22 , 27 - 28 , 34 ] . The adverse reaction rates of the recombinant spike protein vaccine (SCB-2019) reported by Richmond [ 32 ] and the RNA vaccine reported by Walsh [ 33 ] were 34.7% and 39.1%, respectively, and the adverse reaction rates of the RNA vaccine (BNT162b1) reported by Mulligan [ 31 ] and the adenovirus recombinant vector vaccine reported by Zhu [ 35 ] were 52.8% and 73.0%, respectively. Three studies could not obtain the adverse reaction rate [ 19 , 29 - 30 ] . The adverse reactions of all vaccinated testee were mostly mild to moderate, and could be relieved within 24 hours after vaccination. The most common local adverse reaction included pain or tenderness at the injection site [ 19 - 22 , 27 - 35 ] . Fatigue was reported as the most common systemic adverse reaction in 9 studies [ 19 - 20 , 22 , 28 - 29 , 31 , 33 - 35 ] . In addition, fever was reported as the most common systemic adverse reaction in 2 studies [ 21 , 27 ] , and 2 studies reported somatic pain as the most common systemic adverse reaction [ 30 , 32 ] ( Table 4 ).
Effectiveness and safety of vaccines
2.4.2. Dose difference
The difference in injection dose is an important factor affecting the immunogenicity and safety of the vaccine. A total of 9 studies [ 21 - 22 , 27 - 29 , 32 - 35 ] found significant differences in GMT and seroconversion rates obtained from testee with different doses of vaccination, 8 of which [ 20 - 22 , 28 - 29 , 31 , 34 - 35 ] found that GMT increased, and 4 [ 22 , 28 - 29 , 32 ] found that the seroconversion rate of testee increased with the increase of vaccine dose, but the incidence of adverse reactions also increases relatively [ 22 , 28 - 29 , 32 ] . Therefore, when the clinical trial entered Phase III, the researchers set the medium dose as the standard dose of the vaccine [ 19 - 20 ] .
2.4.3. Difference of age
Four studies specifically recruited the elderly 60 years and older, and conducted a special subgroup analysis in the results. Richmond [ 32 ] reported that the GMT range measured by the micro-neutralization test in the elderly group was 1567-3625, which was lower than 2510-4452 in the 18-59-year-old group. The incidence of systemic adverse reactions in the elderly after the first injection was 17%, which was lower than 38% in the 18-59 years-old group. Xia [ 27 ] also reported that the GMT of the elderly group was lower than that of the 18-59 years-old group, and the seroconversion time was later than that of the 18-59 years-old group. The incidence of systemic adverse reactions in the elderly within 7 days after vaccination was 28.6%, which was lower than 41.7% of the 18-59 years-old group. Polack [ 20 ] and Walsh [ 33 ] also reported similar results. In short, compared with healthy people aged 18 to 59, the GMT detected in the serum was significantly lower in elderly population vaccinated with the same vaccine according to the same procedure, but the incidence of adverse reactions in the elderly population was also significantly lower [ 20 , 27 , 32 - 33 ] .
2.4.4. Differences in vaccination procedures
Although a number of studies designed a comparison of different vaccination procedures, the results of the experiment were complicated. Zhang 's research showed that testee who vaccinated at 2-week intervals got a faster immune response, but a stronger immune response at 4-week intervals [ 34 ] . Che detected a stronger immune response in testee who were vaccinated at 2-week intervals [ 28 ] . Xia also found that the incidence of adverse reactions in testee vaccinated at 2-week intervals was lower than that at 4-week intervals [ 21 ] . In 6 studies that compared single-dose and double-dose vaccination, 4 studies showed that double-dose vaccination produced a stronger immune response than single-dose vaccination [ 19 , 31 , 33 , 35 ] .
2.4.5. Differences of vaccine type
The RNA vaccine (BNT162b2) reported by Polack [ 20 ] and the recombinant adenovirus vector vaccine (ChAdOx1 nCoV-19) reported by Voysey [ 19 ] involved more than 10, 000 people, and two both used relative risk to calculate the effective rate, showing that effective rate of the former was 95% [ 20 ] , and the latter was 70.4% [ 19 ] . Owing to differences in the design, the small sample size, and different outcome indicators of other clinical trials, their effective rates were not yet comparable.
3. Discussion
The system evaluation draws the following conclusions: (1) All candidate vaccines have a good immunogenicity and safety except the vaccine reported by Zhu [ 35 ] . Within 28 days after vaccination, the testee' serum GMT increased significantly, and the seroconversion rate was mostly greater than 80%. The adverse reaction rate of most vaccines was less than 30%, degree was mild to moderate, and symptoms were alleviated within 24 hours. (2) The potency and adverse reaction rate after vaccination were positively related to the dose. Most clinical trials chose the middle dose when the phase III. This might be the result of comprehensive consideration of effectiveness and safety. (3) Under the same conditions, the vaccine had poor immunogenicity to elderly people over 60, but the adverse reaction rate was also low. One of the possible reasons was low immunity of the older. A lot of studies on the tolerance of the elderly population to the vaccine still are needed. In addition, there are currently no published results of clinical trials targeting juveniles. (4) Most studies recommend double-dose vaccination, but the interval needs further study.
However, this systematic review has some limitations: (1) No evidence of the long-term effectiveness and safety of the vaccine. Due to the urgency of vaccine development, most trials only followed up to 28 days after vaccination. Whether neutralizing antibodies can be maintained for a long time and whether there are delayed adverse reactions after vaccination still require a longer period. (2) In order to get more up-to-date evidence, this systematic review also includes preprinted documents, which have not been peer reviewed and some of the data are not available. (3) Only randomized, double-blind, and controlled trials were included, while observational studies, retrospective case analysis, and early animal experiments were all excluded. For example, an open label trial conducted by Anderson [ 36 ] found that mRNA-1273 vaccine had a good safety in the elderly population. Logonov [ 37 ] reported two adenovirus recombinant vector vaccine preparations (rAd26) in a non-random clinical trial (rAd26-S and rAd5-S) had a good safety and immunogenicity in healthy people aged 18 to 60. (4) There were differences in the design of various clinical trials, which made it impossible to compare the advantages and disadvantages of different types of vaccines. For example, Voysey [ 19 ] and Polack [ 20 ] used relative risk to calculate the effective rate. Although the remaining 10 studies have completed the virus neutralization test, the experimental design schemes were quite different [ 21 - 22 , 27 - 29 , 31 - 35 ] . (5) Only Chinese and English documents were searched in this systematic review, and documents published in other languages such as Japanese and French were excluded.
In conclusion, this systematic review summarized the results of clinical trials related to the COVID-19 vaccine, showing that most vaccines had a good safety and effectiveness. It is believed that with the widespread vaccination of COVID-19, it is possible to control and end the global pandemic of COVID-19.
Conflict of interest: The authors have no conflicts of interest to disclose.
新型冠状病毒肺炎(COVID-19)疫情暴发至今已1年余。虽然COVID-19疫情在我国已经得到了有效控制, 但全球整体疫情形势依然严峻。根据世界卫生组织的数据, 截至欧洲中部时间2021年2月15日16 : 05, 全球累计COVID-19确诊病例达到108 579 352例, 累计死亡人数达到2 396 408人 [ 1 ] 。作为全球的重大公共卫生事件, COVID-19疫情成为全人类首要的健康威胁, 世界政治经济文化也受到巨大冲击 [ 2 - 3 ] 。导致COVID-19的严重急性呼吸综合征冠状病毒2(SARS-CoV-2)是以RNA为遗传物质的β属冠状病毒, 通过刺突蛋白结合血管紧张素转化酶2进入细胞 [ 4 - 5 ] 。COVID-19患者的首发症状以发热和干咳多见, 在多脏器损伤中, 肺脏受损最为严重 [ 2 , 5 - 6 ] 。在疫情控制上, 佩戴口罩和保持社交距离已经在中国抗击疫情的实践中被确认为阻断病毒传播最为有效的措施 [ 3 , 7 - 9 ] 。在对COVID-19患者的治疗上, 隔离和对症支持治疗仍占主要地位 [ 5 ] , 而关于抗病毒药物和中药等的疗效还需更多证据的支持 [ 10 - 11 ] 。由于口罩在国外普及率的低下和治疗方案的局限性 [ 12 - 13 ] , 越来越多的希望被寄托在COVID-19疫苗的开发上。根据靶点和技术的不同, 疫苗可以被分为以下几类: 灭活疫苗、重组刺突蛋白疫苗、病毒载体疫苗、RNA疫苗、减毒活疫苗和病毒样颗粒疫苗等 [ 14 - 16 ] 。目前, 已有数百项COVID-19候选疫苗的项目在美国临床试验数据库(clinicaltrials.gov)注册 [ 15 , 17 ] , 数种疫苗的3期临床试验结果予以发表 [ 18 - 22 ] 。截至2021年1月1日, 中、俄、美、英等国家先后批准了本国疫苗在人群中的大规模接种计划。本研究通过系统文献复习及定性分析已发表的COVID-19疫苗临床试验结果, 评估COVID-19疫苗的安全性与有效性。
本系统评价遵循《系统评价和Meta分析的首选报告项目(PRISMA)》中的准则完成 [ 23 - 24 ] 。
1.1. 文献纳入标准
文献纳入标准包括: (1)试验对象为18岁及以上的健康男性或未孕女性; (2)干预措施为接种COVID-19疫苗; (3)试验类型为随机、对照、盲法试验; (4)临床试验结果指标至少包括以下一项或几项: 局部不良反应(疼痛、瘙痒、发红、肿胀和硬结等)、全身不良反应(发热、腹泻、疲劳、恶心/呕吐等)、末次疫苗接种14 d或28 d后以活病毒中和试验测得的中和抗体几何平均滴度(GMT)、血清转化率及其他实验室检测指标。
1.2. 文献排除标准
具备以下条件之一的文献被排除: (1)文献类型为医学新闻、科普文章、非医学类论文、综述、信件、评论、基础研究、病例报告、会议摘要; (2) 无法获取全文或以中文、英文外的第三种语言发表的文献; (3)若两项研究的受试者存在重叠, 则其中之一被排除; (4)若文献的数据被之后发表的文献包含在内, 前者予以排除。
对英文数据库PubMed、Embase、Cochrane图书馆和clinicaltrials.gov数据库进行了检索。检索的中文数据库包括中国知网、万方数据库、中国生物医学文献服务系统和中国临床试验注册中心。为了保证检索结果的全面性, 本系统评价运用布尔运算逻辑, 采取"主题词+自由词"方式进行了检索。主要检索词包括: COVID-19、2019-nCoV、SARS-CoV-2、2019 novel coronavirus、vaccines、vaccination、COVID-19 vaccines、mRNA-1273 vaccine、Ad5-nCoV vaccine、ChAdOx1 COVID-19 vaccine、BNT162 vaccine、controlled clinical trial、randomized controlled trials、controlled clinical trial、random、blind、placebo、trial、Meta等。中文检索词包括新型冠状病毒、新冠肺炎、新型冠状病毒肺炎、疫苗、试验、随机对照试验、随机对照研究、随机对照、随机、元分析、Meta、荟萃等。
1.4. 文献筛选和资料提取
文献筛选和资料提取工作由两位研究者独立完成。若结果汇总时出现分歧, 由两位研究者讨论处理或交由第3位研究者决定。在数据库中获得的所有检索结果导入NoteExpress(武汉大学图书馆版)软件中, 使用软件的查重功能机械地去除重复文献。然后通过阅读标题和摘要完成初次筛选, 通过阅读全文完成二次筛选。在第二次筛选中, 每一篇文献被剔除的原因均被记录。所提取数据包括: 第一作者、疫苗类型、接种剂量、接种间隔时间、受试者人数及基线特征(种族、性别比例、年龄范围或平均年龄)、研究设计方案、局部和全身不良反应、实验室检查指标, 以及基金、赞助商和注册号等。
1.5. 方法学质量评价
依据Cochrane系统评价手册评估偏倚风险 [ 25 - 26 ] 。
本系统评价的主要结果包括疫苗的安全性和有效性。评估安全性的指标包括局部不良反应(疼痛、瘙痒、红肿、硬结等)及全身不良反应(咳嗽、腹泻、疲倦、发烧、头痛、恶心/呕吐、瘙痒、肌肉疼痛、关节痛/不适、厌食等)。评估免疫原性的指标包括GMT、血清转化率、IgG或其他特异性抗体对受体结合域的反应。
2.1. 文献检索结果
检索了截至2020年12月31日之前发表的所有相关文献, 共得到753篇。经过筛选后纳入13篇 [ 19 - 22 , 27 - 35 ] 进入本系统评价。文献筛选的具体流程见 图 1 。
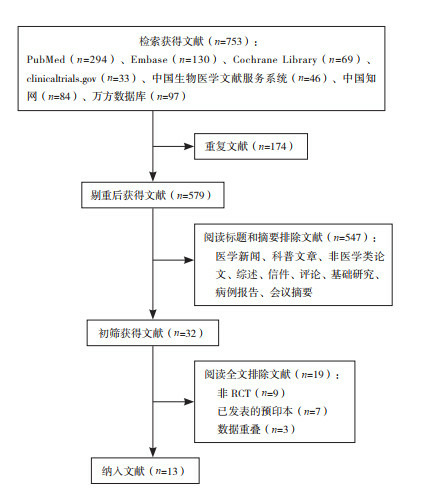
2.2. 纳入研究的方法学质量评价
纳入的13项研究 [ 19 - 22 , 27 - 35 ] 均采用了随机对照的方法, 其中10项 [ 21 - 22 , 27 - 32 , 34 - 35 ] 实施了双盲法, 2项 [ 20 , 33 ] 实施了单盲法, 1项 [ 19 ] 在不同试验地点分别使用了单盲法和双盲法; 所有试验均隐藏了分配方案; 9项 [ 19 , 22 , 27 , 29 - 31 , 33 - 35 ] 数据不完整或选择性报告, 其中2项 [ 22 , 29 ] 预印本缺失数据较多, 其余7项 [ 19 , 27 , 30 - 31 , 33 - 35 ] 缺失个别数据; 9项 [ 19 - 20 , 22 , 29 - 32 , 34 - 35 ] 存在其他类型偏倚, 如Keech等 [ 30 ] 在试验设计中未做病毒中和试验。总的来讲, 所纳入文献的偏倚风险较低。见 图 2 和 表 1 。
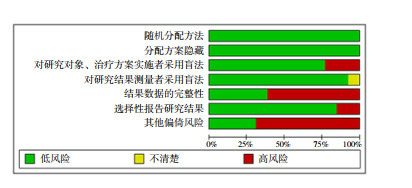
纳入研究的方法学质量评价
2.3. 纳入研究的基本特征
所纳入的13项研究均为随机、盲法、对照试验, 共涉及灭活疫苗5种 [ 21 - 22 , 27 - 29 , 34 ] 、重组刺突蛋白疫苗2种 [ 30 , 32 ] 、RNA疫苗2种 [ 20 , 31 , 33 ] 和腺病毒载体疫苗2种 [ 19 , 35 ] , 疫苗特性、开发者等信息见 表 2 。有6项研究比较了疫苗单剂量与双剂量接种的效应 [ 19 , 27 , 30 - 31 , 33 , 35 ] 。大部分研究比较了以2周、3周或4周为间隔注射两剂疫苗的差别。大部分研究也比较了低、中、高不同注射剂量的差别。所有试验的参与者均为成年人, 有5篇文献报道了疫苗在老年人群体中的结果 [ 19 - 20 , 32 - 33 , 35 ] 。所纳入研究参与者的基线特征见 表 3 。
纳入研究的试验设计和开发者
2.4. 定性分析结果
2.4.1. 疫苗的有效性和安全性.
在10项研究中, 受试者的28 d血清转化率超过80% [ 21 - 22 , 27 - 34 ] ;在两项万人规模的临床试验中, Polack等 [ 20 ] 报道的RNA疫苗(BNT162b2)取得了95%的有效率, Voysey等 [ 19 ] 报道的腺病毒重组载体疫苗(ChAdOx1 nCoV-19)取得了70.4%的有效率; Zhu等 [ 35 ] 报道的腺病毒重组载体疫苗在受试者中的28 d血清转化率低于60%。见 表 4 。
在6项研究中, 志愿者在接种疫苗后的28 d内不良反应发生率低于30% [ 20 - 22 , 27 - 28 , 34 ] ;Richmond等 [ 32 ] 报道的重组刺突蛋白疫苗(SCB-2019)和Walsh等 [ 33 ] 报道的RNA疫苗的不良反应率分别为34.7%和39.1%;Mulligan等 [ 31 ] 报道的RNA疫苗(BNT162b1)和Zhu等 [ 35 ] 报道的腺病毒重组载体疫苗的不良反应率分别为52.8%和73.0%;3项研究无法获取不良反应率 [ 19 , 29 - 30 ] 。所有疫苗接种的受试者发生不良反应事件绝大部分都是轻度到中度, 且在接种后24 h内可缓解; 所有疫苗接种最常见的局部不良反应均为注射部位疼痛或压痛 [ 19 - 22 , 27 - 35 ] ;疲劳在9项研究中被报道为最常见的系统性不良反应 [ 19 - 20 , 22 , 28 - 29 , 31 , 33 - 35 ] 。此外, 发热在2项研究中被报道为最常见的系统性不良反应 [ 21 , 27 ] , 也有2项研究报道躯体痛为最常见的系统性不良反应 [ 30 , 32 ] 。见 表 4 。
2.4.2. 剂量差异的影响
注射剂量的不同是影响疫苗免疫原性和安全性的重要因素。共有9项研究 [ 21 - 22 , 27 - 29 , 32 - 35 ] 发现接受不同剂量疫苗接种的受试者获得的GMT和血清转化率存在显著性差异, 其中8项 [ 20 - 22 , 28 - 29 , 31 , 34 - 35 ] 发现GMT随着疫苗剂量的增加而增加, 4项 [ 22 , 28 - 29 , 32 ] 发现受试者血清转化率随疫苗剂量的增加而增加。但随着接种剂量的加大, 不良反应的发生率也相对增加 [ 22 , 28 - 29 , 32 ] 。因此, 当临床试验进入Ⅲ期阶段, 研究者将中等剂量设定为疫苗的标准剂量 [ 19 - 20 ] 。
2.4.3. 年龄差异的影响
有4项研究专门招募了60岁及以上的老年人群, 并在结果中进行了专门的亚组分析。Richmond等 [ 32 ] 报道使用微量中和试验在老年人组测得的GMT范围为1 567~3 625, 低于18~59岁组的2 510~4 452;而老年人在第1次注射后的全身不良反应发生率为17%, 低于18~59岁组的38%。Xia等 [ 27 ] 也报道老年人组GMT低于18~59岁组, 且达到血清转化时间晚于18~59岁组; 而老年人在接种后7 d内的全身不良反应发生率为28.6%, 低于18~59岁组的41.7%。Polack等 [ 20 ] 和Walsh等 [ 33 ] 两项研究也报道了相似结果。总之, 相比于18~59岁的健康人群, 老年人群按照相同的程序接种同种疫苗后, 血清中所检测到的GMT显著偏低, 但相应地老年人群中不良反应发生率也显著偏低 [ 20 , 27 , 32 - 33 ] 。
2.4.4. 接种程序差异的影响
虽然多项研究设计了不同接种程序的对比, 但试验结果是复杂的。Zhang等 [ 34 ] 的研究表明, 以2周为间隔接种疫苗的受试者获得了更快的免疫反应, 但以4周为间隔接种疫苗的受试者获得了更强的免疫反应。但Che等 [ 28 ] 在以2周为间隔接种疫苗的受试者中检测到了更强的免疫反应, Xia等 [ 21 ] 也发现以2周为间隔接种疫苗的受试者不良反应发生率低于以4周为间隔接种疫苗的受试者。在6项比较了疫苗的单剂量与双剂量接种的研究中, 4项研究显示疫苗双剂量接种比单剂量接种产生更强的免疫反应 [ 19 , 31 , 33 , 35 ] 。
2.4.5. 疫苗类型差异的影响
Polack等 [ 20 ] 报道的RNA疫苗(BNT162b2)和Voysey等 [ 19 ] 报道的腺病毒重组载体疫苗(ChAdOx1 nCoV-19)受试者人数超过10 000人, 都采用相对危险度计算有效率, 显示前者有效率为95% [ 20 ] , 后者有效率为70.4% [ 19 ] 。其他临床试验的设计存在差异, 受试者规模较小, 结局指标也有所不同, 其有效率尚无法比较。
本系统评价得出以下结论: (1)除了Zhu等 [ 35 ] 报道的疫苗外, 所有候选疫苗都具有良好的免疫原性和安全性。接种后28 d内, 受试者血清GMT显著增加, 血清转化率大多大于80%, 大部分疫苗的不良反应率低于30%, 且以轻到中度为主, 24 h内缓解。(2)接种后产生的效价和不良反应率与剂量呈正相关, 因此, 大部分临床试验进入Ⅲ期阶段后, 选择了中等剂量作为标准剂量, 这可能是对有效性和安全性综合考虑的结果。(3) 相同条件下, 疫苗对60岁以上的老年人的免疫原性较差, 但不良反应率也偏低, 一种可能的解释是这与人体的免疫衰老有关。老年人群对疫苗的耐受性需要继续研究。此外, 目前尚没有针对未成年人的临床试验结果发表。(4)大部分疫苗研究都推荐双剂量接种, 但接种间隔时间需进一步研究。
然而, 本系统评价有一定的局限性: (1)缺乏疫苗的长期有效性和安全性的证据。由于疫苗研发的急迫性, 大部分试验只随访到了接种后28 d, 中和性抗体能否长期维持, 接种疫苗后是否有迟发的不良反应, 仍需要更长时间的随访。(2)为了纳入更多最新证据, 本系统评价也将预印本文献包含在内, 这些文献没有经过同行评议, 且其中一些数据无法获取。(3)本系统评价只纳入了随机、双盲、对照试验, 而观察性研究、回顾性病例分析及早期的动物试验均被排除在外。如Anderson等 [ 36 ] 实施的一项开放标签试验发现mRNA-1273疫苗在老年人群体具有较好的安全性, Logunov等 [ 37 ] 在非随机临床试验中报道了两种腺病毒重组载体疫苗制剂(rAd26-S和rAd5-S)在18~60岁健康人群具有较好的安全性和免疫原性。(4)各项临床试验的设计存在差异, 导致无法对不同类型疫苗的优劣进行比较, 如Voysey等 [ 19 ] 和Polack等 [ 20 ] 采用相对危险度计算有效率, Keech等 [ 30 ] 未做病毒中和试验, 其余10项研究虽然均完成了病毒中和试验, 但试验设计方案差异较大 [ 21 - 22 , 27 - 29 , 31 - 35 ] 。(5)本系统评价只检索了中英文文献, 以日文、法文等其他语言发表的文献被排除在外。
综上所述, 本系统评价总结了COVID-19疫苗相关的临床试验结果, 表明大部分疫苗都具有较好的安全性和有效性。这让我们有理由相信, 随着COVID-19疫苗的广泛接种, 有望控制、终结COVID-19的全球大流行。
利益冲突声明:所有作者均声明不存在利益冲突。
Biographies
Jiang Y, Email: [email protected]
Funding Statement
中央高校基本科研业务费专项资金资助项目(2042020kf1011)
Fundamental Research Funds for the Central Universities (2042020kf1011)
- 1. World Health Organization. WHO coronavirus disease (COVID-19) dashboard[EB/OL]. (2021-02-15)[2021-02-16]. https://covid19.who.int/.
- 2. Sun JM, He WT, Wang LF, et al. COVID-19:epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol Med. 2020;26(5):483–495. doi: 10.1016/j.molmed.2020.02.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Xi JP. Remarks at the National Commendation Conference on COVID-19 (8th September 2020) Qiushi. 2020;(20):4–15. [ Google Scholar ]; 习 近平. 在全国抗击新冠肺炎疫情表彰大会上的讲话(2020年9月8日) 求是. 2020;(20):4–15. [ Google Scholar ]
- 4. Zhu N, Zhang DY, Wang WL, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. National Health Commission of the People's Republic of China The clinical significance of COVID-19 Diagnosis and Treatment Guidelines (Interim version 8) Journal of Tropical Diseases and Parasitology. 2020;13(5):321–328. [ Google Scholar ]; 中华人民共和国国家卫生健康委员会 新型冠状病毒肺炎诊疗方案(试行第八版) 中华临床感染病杂志. 2020;13(5):321–328. [ Google Scholar ]
- 6. Oliveira BA, Oliveira LC, Sabino EC, et al. SARS-CoV-2 and the COVID-19 disease: a mini review on diagnostic methods. Rev Inst Med Trop Sao Paulo. 2020;62:e44. doi: 10.1590/s1678-9946202062044. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Wang J, Pan LJ, Tang S, et al. Mask use during COVID-19:a risk adjusted strategy. Environ Pollut. 2020;266(Pt 1):115099. doi: 10.1016/j.envpol.2020.115099. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Li T, Liu Y, Li M, et al. Mask or no mask for COVID-19:a public health and market study. PLoS One. 2020;15(8):e0237691. doi: 10.1371/journal.pone.0237691. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. General Office of the National Health Commission, PRC Technical guidelines on prevention and control of novel coronavirus infection in medical institutions (First edition) Chinese Journal of Infection Control. 2020;19(2):189–191. [ Google Scholar ]; 中华人民共和国国家卫生健康委员会办公厅 医疗机构内新型冠状病毒感染预防与控制技术指南(第一版) 中国感染控制杂志. 2020;19(2):189–191. [ Google Scholar ]
- 10. Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19:an evaluation of the evidence. Travel Med Infect Dis. 2020;35:101647. doi: 10.1016/j.tmaid.2020.101647. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Pardo J, Shukla AM, Chamarthi G, et al. The journey of Remdesivir: from Ebola to COVID-19. Drugs Context. 2020;9:2020–2024. doi: 10.7573/dic.2020-4-14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Tirupathi R, Bharathidasan K, Palabindala V, et al. Comprehensive review of mask utility and challenges during the COVID-19 pandemic. Infez Med. 2020;28(Suppl 1):57–63. [ PubMed ] [ Google Scholar ]
- 13. Provenzani A, Polidori P. COVID-19 and drug therapy, what we learned. Int J Clin Pharm. 2020;42(3):833–836. doi: 10.1007/s11096-020-01049-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Romero JR, Bernstein HH. COVID-19 vaccines: a primer for clinicians. Pediatr Ann. 2020;49(12):e532–e536. doi: 10.3928/19382359-20201116-01. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Sharma O, Sultan AA, Ding H, et al. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11:585354. doi: 10.3389/fimmu.2020.585354. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Korang SK, Juul S, Nielsen EE, et al. Vaccines to prevent COVID-19:a protocol for a living systematic review with network meta-analysis including individual patient data (The LIVING VACCINE Project) Syst Rev. 2020;9(1):262. doi: 10.1186/s13643-020-01516-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Wang JL, Peng Y, Xu HY, et al. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech. 2020;21(6):225. doi: 10.1208/s12249-020-01744-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2:an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Xia SL, Duan K, Zhang YT, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Pu J, Yu Q, Yin ZF, et al. An in-depth investigation of the safety and immunogenicity of an inactivated SARS-CoV-2 vaccine[J]. medRxiv, 2020. DOI: 10.1101/2020.09.27.20189548.Epubaheadofprint.
- 23. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions[M]. 2nd ed. Chichester, UK: John Wiley & Sons, 2019.
- 26. Cumpston M, Li TJ, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Xia SL, Zhang YT, Wang YX, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Che YC, Liu XQ, Pu Y, et al. Randomized, double-blinded, placebo-controlled phase 2 trial of an inactivated severe acute respiratory syndrome coronavirus 2 vaccine in healthy adults[J]. Clin Infect Dis, 2020. DOI: 10.1093/cid/ciaa1703.Epubaheadofprint.
- 29. Ella R, Vadrevu KM, Jogdand H, et al. A phase 1: safety and immunogenicity trial of an inactivated SARS-CoV-2 vaccine-BBV152[J]. medRxiv, 2020. DOI: 10.1101/2020.12.11.20210419.Epubaheadofprint.
- 30. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/Ⅱ study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Richmond P, Hatchuel L, Dong M, et al. A first-in-human evaluation of the safety and immunogenicity of SCB-2019, an adjuvanted, recombinant SARS-CoV-2 trimeric S-protein subunit vaccine for COVID-19 in healthy adults; a phase 1, randomised, double-blind, placebo-controlled trial[J]. medRxiv, 2020. DOI: 10.1101/2020.12.03.20243709.Epubaheadofprint.
- 33. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Zhang YJ, Zeng G, Pan HX, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.1 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis
Affiliations.
- 1 Department of Epidemiology, School of Public Health, Zhengzhou University, Zhengzhou, Henan, 450001, China.
- 2 Department of Epidemiology, School of Public Health, Zhengzhou University, Zhengzhou, Henan, 450001, China. Electronic address: [email protected].
- PMID: 34800687
- PMCID: PMC8595975
- DOI: 10.1016/j.ijid.2021.11.009
Objective: To estimate the coronavirus disease 2019 (COVID-19) vaccine effectiveness (VE) against concerned outcomes in real-world settings.
Methods: Studies reporting COVID-19 VE from August 6, 2020 to October 6, 2021 were included. The summary VE (with 95% confidence intervals (95% CI)) against disease related to COVID-19 was estimated. The results were presented in forest plots. Predefined subgroup analyses and sensitivity analyses were also performed.
Results: A total of 51 records were included in this meta-analysis. In fully vaccinated populations, the VE against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, COVID-19-related hospitalization, admission to the intensive care unit, and death was 89.1% (95% CI 85.6-92.6%), 97.2% (95% CI 96.1-98.3%), 97.4% (95% CI 96.0-98.8%), and 99.0% (95% CI 98.5-99.6%), respectively. The VE against infection in the general population aged ≥16 years, the elderly, and healthcare workers was 86.1% (95% CI 77.8-94.4%), 83.8% (95% CI 77.1-90.6%), and 95.3% (95% CI 92.0-98.6%), respectively. For those fully vaccinated against infection, the observed effectiveness of the Pfizer-BioNTech vaccine was 91.2% and of the Moderna vaccine was 98.1%, while the effectiveness of the CoronaVac vaccine was found to be 65.7%.
Conclusions: The COVID-19 vaccines are highly protective against SARS-CoV-2-related diseases in real-world settings.
Keywords: COVID-19; Effectiveness; Real-world; SARS-CoV-2; Vaccine.
Copyright © 2021 Zhengzhou University. Published by Elsevier Ltd.. All rights reserved.
Publication types
- Meta-Analysis
- COVID-19 Vaccines*
- Hospitalization
- Vaccine Efficacy
- COVID-19 Vaccines

IMAGES