An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Enzyme immobilization: an update
Ahmad abolpour homaei, reyhaneh sariri, fabio vianello, roberto stevanato.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2013 Jun 5; Accepted 2013 Jul 31; Collection date 2013 Oct.
Compared to free enzymes in solution, immobilized enzymes are more robust and more resistant to environmental changes. More importantly, the heterogeneity of the immo-bilized enzyme systems allows an easy recovery of both enzymes and products, multiple re-use of enzymes, continuous operation of enzymatic processes, rapid termination of reactions, and greater variety of bioreactor designs. This paper is a review of the recent literatures on enzyme immobilization by various techniques, the need for immobilization and different applications in industry, covering the last two decades. The most recent papers, patents, and reviews on immobilization strategies and application are reviewed.
Keywords: Enzyme immobilization, Biocatalyst, Enzyme reuse
Structural abstract. A summary of enzyme immobilization techniques investigated in this review work showing their advantage, disadvantages, and various applications.
Introduction
The relationship between humans and enzymes has evolved over time. Even during historical times, where there was no concept of enzymes, ancient Egypt people produced beer and wine by enzymatic fermentation. After several thousand years, enzymatic studies have significantly progressed. Enzymes are proteins that accelerate many biochemical and chemical reactions. They are natural catalysts and are ubiquitous, in plants, animals, and microorganisms, where they catalyze processes that are vital to living organisms. The growing knowledge and technique improvement about protein extraction and purification lead to the production of many enzymes at an analytical grade purity for research and biotechnological applications. Enzymes are intimately involved in a wide variety of traditional food processes, such as cheese making, beer brewing, and wine industry. Recent advances in biotechnology, particularly in protein engineering, have provided the basis for the efficient development of enzymes with improved properties. This has led to establishment of novel, tailor-made enzymes for completely new applications, where enzymes were not previously used.
Application of enzymes in different industries is continuously increasing, especially during the last two decades. Applications of enzymes in food industries include baking [ 1 ], dairy products [ 2 , 3 ], starch conversion [ 4 ] and beverage processing (beer, wine, fruit and vegetable juices). In textiles industries, enzymes have found a special place due to their effect on end products [ 5 ]. In industries such as pulp and paper making [ 6 ] and detergents [ 7 ], the use of enzymes has become an inevitable processing strategy when a perfect end product is desired. Application of enzymes in more modern industries including biosensors is improving rapidly due to the specificity of enzymes which is of prime importance in biosensor [ 8 ]. Many other important industries including health care and pharmaceuticals [ 9 ] and chemical [ 10 ] manufacture are increasingly taking advantages of these natures amazing catalysts. During the last few years, enzymes have widely been used in biofuels, such as biodiesel and ethanol [ 11 ].One of the best use of enzymes in the modern life is their application in treatment of wastes in general [ 12 ] and especially for solid wastes treatment [ 13 ] and waste water purification [ 14 ].
In some cases, industrial applications of enzymes in organic solvents are also developed [ 15 ]. Moreover, enzymes are produced from renewable raw materials and are completely biodegradable. In addition, the mild operating conditions of enzymatic processes mean that they can be operated in relatively simple and totally controlled equipment. In short, they reduce environmental drawbacks of manufacturing by reducing the consumption of energy and chemicals and concomitant generation of wastes.
However, all these desirable characteristics of enzymes and their widespread industrial applications are often hampered by their lack of long-term operational stability and shelf-storage life and by their cumbersome recovery and re-use. These drawbacks can generally be overcome by immobilization of enzymes. In fact, a major challenge in industrial bio-catalysis is the development of stable, robust, and preferably insoluble biocatalysts.
Historical background
The first scientific observation that led to the discovery of immobilized enzymes was made in 1916 [ 1 ]. It was demonstrated that invertase exhibited the same activity when absorbed on a solid, such as charcoal or aluminum hydroxide, at the bottom of the reaction vessel as when uniformly distributed throughout the solution. This discovery was later developed to the currently available enzyme immobilization techniques.
Early immobilization techniques provided very low enzyme loadings, relative to available surface areas. During 1950s and 1960s, different covalent methods of enzyme immobilization were developed. Continuing from 1960s, to date more than 5,000 publications and patents have been published on enzyme immobilization techniques. Several hundred enzymes have been immobilized in different forms and more than a dozen immobilized enzymes; for example, penicillin G acylase, invertase, lipases, proteases, etc. have been used as catalysts in various large-scale processes. While enzyme immobilization has been studied for a number of years, the appearance of recent published research and review papers indicates a continued interest in this area [ 16 – 18 ]. According to PubMed database only in the first 6 months of 2010, many hundreds papers on enzyme immobilization have been published.
Today, in many cases immobilized enzymes have revealed highly efficient for commercial uses. They offer many advantages over enzymes in solution, including economic convenience, higher stability, and the possibility to be easily removed from the reaction mixture leading to pure product isolation. An immobilized enzyme is, therefore, attached to an inert, organic, or inorganic or insoluble material, such as calcium alginate or silica. Furthermore, the attachment of an enzyme to a solid support can increase its resistance to various environmental changes such as pH or temperature [ 19 ].
Immobilization strategies
Despite of many advantages in the use of enzymes compared to traditional catalysts, there are few practical problems associated with their utilization in industrial applications. Enzymes are generally expensive, which means that the cost of their isolation and purification is many times higher than that of ordinary catalysts. Being protein in structure, they are also highly sensitive to various denaturing conditions when isolated from their natural environments. Their sensitivity to process conditions, such as temperature, pH, and substances at trace levels, can act as inhibitors which add to their costs. On the other hand, unlike conventional heterogeneous chemical catalysts, most enzymes operate dissolved in water in homogeneous catalysis systems, leading to product contamination and ruling out their recovery, for reuse, in the active form from most of the reaction mixtures.
One of the most successful methods proposed to overcome these limitations is the use of an immobilization strategy (van de Velde 2002). Immobilization is a technical process in which enzymes are fixed to or within solid supports, creating a heterogeneous immobilized enzyme system. Immobilized form of enzymes mimic their natural mode in living cells, where most of them are attached to cellular cytoskeleton, membrane, and organelle structures. The solid support systems generally stabilize the structure of the enzymes and, as a consequence, maintain their activities. Thus, as compared to free enzymes in solution, immobilized enzymes are more robust and more resistant to environmental changes. In addition, heterogeneous immobilized enzymes systems allow the easy recovery of both enzymes and products, multiple reuse of enzymes, continuous operation of enzymatic processes, rapid termination of reactions, and greater variety of bioreactor designs. On the other hand, compared with free enzymes, most commonly immobilized enzymes show lower activity and, generally, higher apparent Michaelis constants because of a relative difficulty in accessing the substrate [ 20 ].
In recent years, a wide range of interest and high attention has been directed toward exploring the potential of immobilized enzymes [ 21 ]. Compared to their free forms, immobilized enzymes are generally more stable and easier to handle. In addition, the reaction products are not contaminated with the enzyme (especially useful in the food and pharmaceutical industries), and in the case of proteases, the rate of the autolysis process can be dramatically reduced upon immobilization [ 22 ].
These alterations result from structural changes introduced into the enzyme molecule by the applied immobilization procedure and from the creation of a microenvironment in which the enzyme works, different from the bulk solution. The result would be a pure product not contaminated with other environmental ingredients and easy to be isolated from the solution. The attached enzyme is then ready for the subsequent reactions without the need for repeated, time-consuming, and costly extraction and purification procedures. Enzymes may be immobilized by a variety of methods, which may be broadly classified as physical, where weak interactions between support and enzyme exist, and chemical, where covalent bonds are formed between the support and the enzyme.
In particular, the development and applications of site-selective protein immobilization have undergone significant advances in recent years. Notably, advances in organic chemistry and molecular biology have resulted in the development of some very powerful, efficient, site-specific, and important applications of anchoring proteins onto supports. These have been followed by the development of functional protein microarrays, biosensors, and continuous flow reactor systems. The advent of high-density array printing technology and improved methods for high-throughput production and purification of large numbers of proteins have, in recent years, allowed the preparation and exploitation of protein microarrays. However, many of the applications reported have relied on protein attachment methods that result in non-specific immobilization, either covalently through amine, aldehyde, and epoxy-derivatized surfaces or through adsorption on nitrocellulose-, hydrogel-, or polylysine-coated slides. A number of more selective approaches have been demonstrated employing affinity reagents that bind specific epitopes or tags on proteins and expose them in a correctly orientated manner, such as nickel nitrilotriacetic acid (Ni-NTA)-coated slides, which are used to bind histidine-tagged proteins and streptavidin (or avidin). Figure 1 schematically explains the mechanism by which a protein is first tagged by two molecules of histidine followed by interaction with a Ni-NTA nanogold. It can be seen in this figure that this type of interaction can only provide non-covalent attachment [ 3 ].
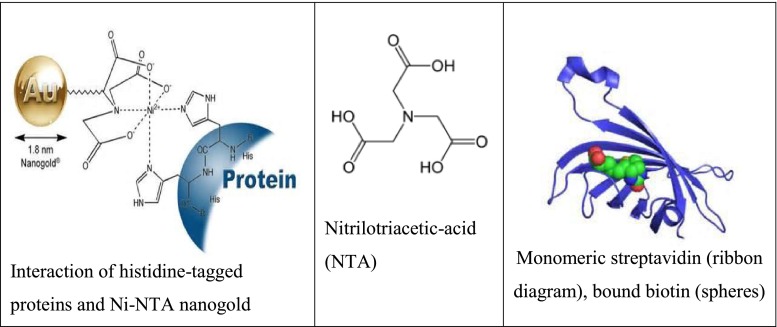
Selective approaches employing affinity reagents
Bioconjugation is an important aspect of the biological sciences and critical to the applications discussed above; as a result, many methods have been described through recent years. Of these, a small number have become widely applied due to their ease of use, flexibility, and reagent commercial availability. In general, these methods rely on physicochemical adsorption phenomena or on functional groups that are naturally present in proteins. They are, therefore, extremely straightforward to employ and applicable to all proteins, both native and modified.
Modes of immobilization
Traditionally, four methods are used for enzyme immobilization, namely (1) non-covalent adsorption and deposition, (2) physical entrapment, (3) covalent attachment, and (4) bio-conjugation (Fig. 2 ). Support binding can be physical or chemical, involving weak or covalent bonds. In general, physical bonding is comparatively weak and is hardly able to keep the enzyme fixed to the carrier under industrial conditions. The support can be a synthetic resin, an inorganic polymer such as zeolite or silica, or a biopolymer. Entrapment involves inclusion of an enzyme in a polymer network (gel lattice) such as an organic polymer or a silica sol–gel, or a membrane device such as a hollow fiber or a microcapsule. Entrapment requires the synthesis of the polymeric network in the presence of the enzyme. The last category involves cross-linking of enzyme aggregates or crystals, using a bifunctional reagent, to prepare carrier-free macroparticles [ 23 ].
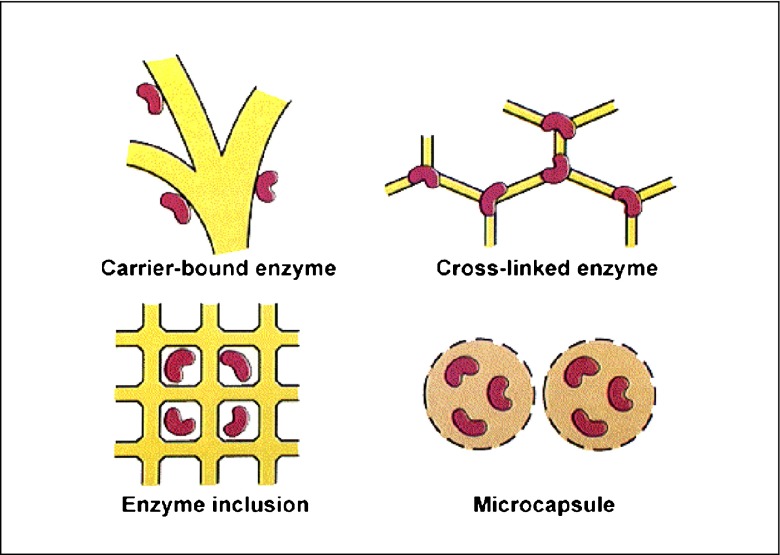
Some methods used for enzyme immobilization
Many other methods, which are either original combinations of the ones listed, sometimes specific for a given support or enzyme have been developed. However, no single method and support is the best for all enzymes and their various applications. This is because of the widely different chemical characteristics and composition of enzymes, the different properties of substrates and products, and the various uses of the product. However, all of the methods may present a number of advantages and drawbacks. Adsorption is simple, cheap, and effective but frequently reversible; covalent attachment and cross-linking are effective and durable, but expensive and easily worsening the enzyme performance; diffusional problems are inherent in membrane reactor-confinement, entrapment, and micro-encapsulations.
Protein adsorption
Non-covalent methods of protein immobilization are widely employed and involve either passive adsorption onto hydrophobic surfaces or electrostatic interactions with charged surfaces. The use of nitrocellulose membranes or polylysine-coated slides for electrostatic binding is perhaps the most widely familiar. As noted above, the major advantage of this kind of immobilization is that neither additional coupling reagents nor modification of the protein of interest is required. However, non-covalent immobilization typically involves relatively weak and reversible interactions. As a result, proteins can leach out from the support, which in turn results in loss of activity with time and contamination of the surrounding media. This has implications in the overall robustness and recyclability of systems, particularly when used in analytical assays and sensor devices. It is also well-known that absorption of protein onto surfaces often results in conformational changes and denaturation of proteins that can lead to massive losses of protein activity. Furthermore, since there is no control over the packing density of the immobilized proteins, their activity may be further reduced by excessive crowding.
Protein immobilization by absorption on mesoporous silicates
In the past decade, much work has been undertaken in the synthesis of mesoporous silicates and in the immobilization of enzymes onto these supports. In 1992, the Mobil research group [ 24 ] discovered a family of mesoporous silicates which had a narrow pore size distribution, amorphous silica surfaces, and pore sizes in the range of 20–300 Å. These new regular repeating mesoporous structures offered the possibility of adsorbing or entrapping large molecules within their pores. Since their development, these materials have promised an improvement about catalytic research of immobilized enzymes. It was anticipated that mesoporous silicates would provide a sheltered protected environment in which reactions with selected substrates could proceed. Following their initial discovery, mesoporous structures have been synthesized using cationic, neutral, and block copolymer surfactants. These copolymers contain organic functional groups and metals located within their framework or grafted onto their surface and have been used as a scaffold to develop mesoporous carbon materials [ 2 ].
Mesoporous silicates possess a number of additional attributes which make them attractive candidates for the immobilization of proteins. It is possible to chemically modify their surfaces with various functional groups, enabling electrostatic attraction or repulsion between the mesoporous silicate support and the biological molecule of interest. As a result of their silicate inorganic framework, mesoporous silicates are chemically and mechanically stable and are resistant to microbial attack.
Materials, such as silica sol–gels, display similar stability to mesoporous silicates and have been used to encapsulate proteins for the development of biosensors. However, sol–gels suffer from the disadvantage of possessing a highly variable pore size distribution (10–400 Å). More importantly, their preparation can involve the use of harsh conditions or reagents, which can cause denaturation of proteins and are detrimental to enzyme activity. Using mesoporous silicates, protein encapsulation occurs after the synthesis of the support, avoiding this difficulty [ 25 ].
Protein immobilization on polyketone polymer by hydrogen bonds
An innovative immobilization process has been recently proposed [ 46 ] which utilizes polyketone polymer, a completely new support. Polyketone polymer –[–CO-CH 2 CH 2 –] n –, obtained by copolymerization of ethane and carbon monoxide, has been utilized for immobilization of three different enzymes, one peroxidase from horseradish and two amine oxidases from bovine serum and lentil seedlings. The easy immobilization procedure was carried out in diluted aqueous buffer, gently mixing the proteins with the polymer. No bi-functional agents or spacer arms are required for the immobilization, which occurs exclusively via a large number of hydrogen bonds between the carbonyl groups of the polymer and the –NH groups of the polypeptidic chain (Fig. 3 ).
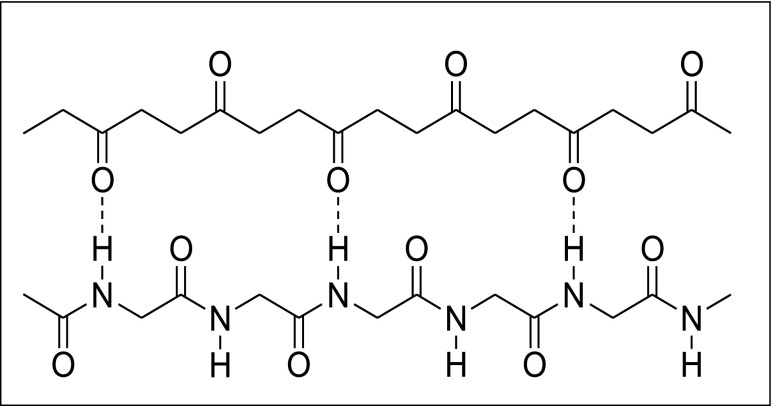
Hydrogen bonds between carbonyl groups and the –NH groups of the polypeptidic chain
Experiments demonstrate a high binding capacity and extraordinary stable immobilization, at least for amine oxidases. Moreover, activity measurements demonstrate that immo-bilized enzymes retain their fully catalytic characteristics, where only a small increase of K M value is observed. The peroxidase activated polymer was used as active packed bed of an enzymatic reactor for continuous flow conversion and flow injection analysis of hydrogen peroxide containing solutions.
Classical covalent immobilization methods
For more stable attachment, the formation of covalent bonds is required, and these are generally formed through reaction with functional groups present on the protein surface. In common with non-covalent adsorption, these methods can be used on unmodified proteins since they rely only on naturally present functional groups. For example, the exposed amine groups of lysine residues readily react with supports bearing active esters, with the most common being N -hydroxysuccinimide (NHS) esters, to form stable amide bonds. However, one disadvantage of using NHS esters is that they are unstable in aqueous conditions, and thus, the attachment of proteins in aqueous buffers will compete with ester hydrolysis, possibly resulting in a modest immobilization yield. As an alternative, aldehyde groups can be coupled with exposed amino groups followed by reduction using sodium cyanoborohydride, or other reagent, to form a stable secondary amine linkage.
The nucleophilicity of the amine group also allows reaction with epoxide-functionalized materials (diglycidyl ethers). These epoxides have the advantage of being relatively stable to hydrolysis at neutral pH, which allows easy handling of the materials but can result in slow or incomplete coupling. Moreover, it is necessary to have supports with the highest degree of epoxy groups, in conditions where they may be very stable to permit long enzyme–support reactions and having the shortest possible spacer arms (mainly in the third generation of epoxy supports) to effectively freeze the enzyme structure. New heterofunctional supports or new uses of the above-described supports may speed up the implementation of many industrial bioprocesses, at present hampered by the lack of suitable biocatalysts [ 4 , 5 ].
Cysteine residues, bearing the thiol group, are also often employed for protein immobilization and readily undergo conjugate addition with unsaturated carbonyls (e.g., maleimides) to form stable thioether bonds. It has been shown that maleimide groups strongly favor conjugate addition with thiols at physiological pH (6.5–7.5) since under these conditions amines are predominantly protonated and un-reactive (Fig. 4 ). As proteins generally have very few surface-exposed cysteine residues, it is possible to achieve site-selective immobilization, especially if the protein of interest can be engineered to remove all but one surface cysteine residue or to insert a single cysteine on the surface where none previously existed. The nucleophilicity of the thiol group also means that it can react with epoxides and NHS esters, although in practical terms this latter reaction is relatively slow and the resultant thioester moiety is susceptible to hydrolysis.
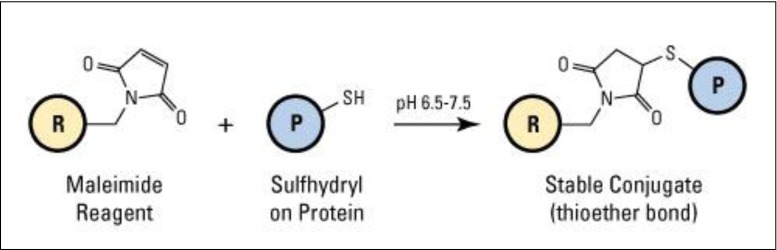
Reaction of maleimide forming chemical conjugation to a sulfhydryl
As regard aspartic and glutamic acid residues, the generic method in which they can be used for immobilization is by conversion to their corresponding active esters in situ with a carbodiimide coupling agent and an auxiliary nucleophile. The most commonly used example of the former is 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, while NHS is widely used to generate the NHS ester on the protein. This active ester can then react with amine-bearing supports. The advantage of this combination of reagents is that both are water-soluble and may be used in aqueous media, although the instability of carbodiimides and the subsequently generated active esters under these conditions means that the reaction yields are often rather low. There is also the risk that the NHS esters formed on the protein molecule may then couple to other protein molecules to give poorly defined polymers.
Oxidative cleavage of the 1,2-diols on the oligosaccharides (usually with periodate) generates aldehyde moieties that can then be used for attachment in a semispecific manner to hydrazine or hydroxylamine functionalized supports via their respective hydrazone or oxime. In addition to antibodies, this strategy has also been applied to a range of other proteins that feature post-translational glycosylation, including several protease and oxidase enzymes [ 26 ]. However, it should be noted that, apart from the multiple potential attachment points on a polysaccharide chain, random orientation may also occur if the desired protein has more than one site of glycosylation on its surface.
In recent years, several selective immobilization methods able to proceed under mild physiological conditions have received increasing attention. Typically these methods rely on the labeling of the protein of interest with an azide moiety. In the Staudinger ligation, the reaction of an azide with a phosphine forms an intermediate iminophosphorane (aza-ylide) that can then react with electrophiles to give a variety of products [ 27 , 28 ]. The generation of an iminophosphorane is typically followed by reaction with an ester to form a stable amide bond. In the first version of this approach, the electrophilic ester is incorporated into the phosphine to give a final product with the phosphine oxide attached to the linkage. The Staudinger ligation has been exploited in a large number of applications from the labeling of proteins and cell-surface glycans to the chemical synthesis of proteins. More recently, the Staudinger ligation has also been applied for the immobilization of peptides and proteins [ 29 ]. In all cases, a two-step process was required: labeling of the protein of interest with the azide followed by the immobilization reaction. Thus, in order to achieve site-selective immobilization, a method for selectively introducing the required azide moiety must first be employed, such as an enzymatic reaction that recognizes specific protein sequences. The general strategy is, therefore, first to introduce the DNA sequence coding for the tag adjacent to the gene encoding for the protein of interest. Subsequently, expression of the engineered synthetic gene then yields a fusion protein of the original protein of interest attached to the tagging protein or peptide containing the attachment site. This fusion protein is then used for the immobilization procedure.
Another method of selective immobilization is derived from the Huisgen 1,3-dipolar cycloaddition of azides with an alkyne, where the covalent link is formed through the formation of a 1,4-disubstituted 1,2,3-triazole [ 30 ]. This reaction has been popularized as “click” chemistry [ 31 ], and in the most well-known version, a terminal alkyne and azide are reacted with Cu(I) catalysts to give near-quantitative conversions to the triazole. The alkyne moiety is also rarely present in biological pathways and adds further versatility to this reaction since the alkyne may be introduced to the biomolecule instead of the azide. This type of “click” chemistry has been used in a variety of applications to enable the attachment of biomolecules bearing either the azide or alkyne with various polymers, fluorophores, or biochemical labels functionalized with their counterpart moieties [ 32 ]. Similar to the Staudinger chemistry, the site-selective attachment of proteins also requires its use in tandem with an enzymatic site-selective labeling method.
Another related family of reactions is “photoclick chemistry,” which relies on photoirradiation to trigger pyrazoline formation between tetrazoles and alkenes [ 33 ]. An added advantage of this method is that the newly formed heterocycle is fluorescent, enabling the monitoring of the reaction progress. Although these are interesting developments, at the moment these have not yet been applied for the purpose of protein immobilization (Figs. 5 and 6 ).
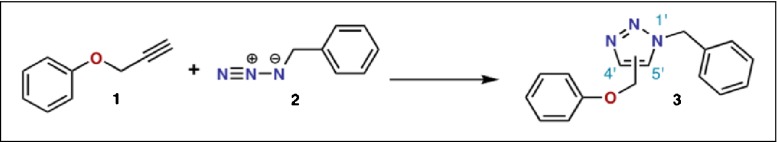
The “click” chemistry reaction of alkyne ( 1 ) with azide ( 2 ) to form a 1,4-disubstituted 1,2,3-triazol
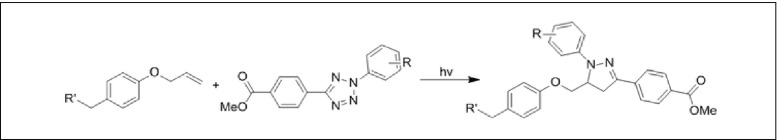
The “photoclick chemistry” between tetrazoles and alkenes to form pyrazoline
Physical entrapment
The best means of avoiding any negative influence on the structure of an enzyme is to encapsulate it. Proteins or enzymes can be extremely fragile and easily aggressed by external agent such as proteases. Encapsulation of these fragile macromolecules is a possible strategy for preventing their aggression and denaturation. A well-established technique to encapsulate biological species such as enzymes, antibodies, and other proteins in a functional state is based on the sol–gel chemistry method.
Sol–gels are silica materials that are highly porous and readily prepared. The sol–gel is a chemically inert glass that can be shaped in any desired way. It can be designed to be thermally and mechanically very stable, but the standard sol–gel is brittle. Sol–gels have been used extensively in the immobilization of proteins and in particular for the development of biosensors. Although sol–gels are porous, diffusion of substrate to the enzyme can be restricted and care has to be taken to minimize this effect. The synthesis of sol–gels is relatively mild for enzymes. In the first step, a tetra-alkoxysilane is hydrolyzed via acid catalysis. Hydrolysis is followed by condensation where the sol is formed. This is a mixture of partially hydrolyzed and partially condensed. All the pores of this gel are filled with water and alcohol; it is therefore known as aquagel. When the aquagel is dried by evaporation, a xerogel is obtained. Due to the action of capillary forces during the evaporation process, the aquagel shrinks and part of the structure collapses. The xerogel consequently does not have the same structure as the aquagel. To avoid such capillary action, the water in the aquagel can be exchanged with acetone and then with supercritical carbon dioxide. On evaporation of the carbon dioxide, the structure of the aquagel is maintained and a brittle aerogel is obtained. In this manner, hydrophilic aqua-, xero-, and aerogels are made. By adding alkyltrialkoxysilanes to the synthesis mixture, sol–gels with a hydrophobic surface can be obtained. Overall the sol–gel method can generate gels with very different properties [ 6 ].
Many encapsulation methods have been developed, and this task can be achieved with lipid vesicles (also called liposomes ), which are polymolecular aggregates formed in aqueous solution on the dispersion of certain bilayer-forming amphiphilic molecules, such as phospholipids. Under osmotically balanced conditions, the vesicles are spherical in shape and contain one or more (concentric) lamellae that are composed of amphiphilic molecules. These shells are curved and self-closed molecular bilayers in which the hydrophobic part of the amphiphiles forms the hydrophobic interior of the bilayer and the hydrophilic part (the polar head group) is in contact with the aqueous phase. The interior of the lipid vesicles is an aqueous core, the chemical composition of which corresponds in a first approximation to the chemical composition of the aqueous solution in which the vesicles are prepared. Depending on the method of preparation, lipid vesicles can be multi-, oligo-, or unilamellar, containing many, a few, or one bilayer shell(s), respectively. The diameter of the lipid vesicles may vary between about 20 nm and a few hundred micrometers. Lipid vesicles are generally not a thermodynamically stable state of amphiphiles and do not form “spontaneously” (without input of external energy); they are only kinetically stable, kinetically trapped systems. The physical properties of lipid vesicles depend on how and under which conditions lipid vesicles of a certain amphiphile (or of a mixture of amphiphiles) are prepared. The mean size, the lamellarity, and the physical stability of the vesicles not only depend on the chemical structure of the amphiphiles used, but in general particularly on the method of vesicle preparation. Physical instabilities of lipid vesicle systems involve vesicle aggregation and fusion, possibly leading to vesicle precipitation and flat bilayer formation.
The basic principles for the preparation of enzyme-containing lipid vesicles in general do not so depend on the chemical structure of the amphiphiles used. Care should be taken to that all mechanical treatments used during vesicle preparation, such as lipid dispersion, sonication, or filtration through polycarbonate membranes, have to be carried out above the transition temperature ( T m ). Below T m , the saturated hydrophobic chains exist predominately in a rigid, extended all trans conformation, similar to their crystalline state. Above T m , the chains are rather disordered, making the bilayer fluid (mechanically treatable), characterized by increased lateral and rotational lipid diffusion rather similar to a liquid. It is this fluid state of the lipids in biological membranes which represents a fluid matrix for membrane proteins.
The involvement of the highly reproducible extrusion technique, particularly with polycarbonate membranes containing pores with a mean diameter of tenth of nanometers, results in homogenous and mainly unilamellar vesicles. In addition to the high degree of reproducibility of the preparation, one advantage of the extrusion technique is certainly the fact that no organic solvent is involved. One possible disadvantage is the shear force present when the vesicles are squeezed through the approximately cylindrical pores, which can inactivate the enzyme. The encapsulation yield at a fixed lipid concentration may also be dependent on the enzyme concentration and on the nature and ionic strength of the buffer used. In comparison with liposomes prepared by the extrusion technique, vesicles prepared by dehydration–rehydration are generally not unilamellar and not monodisperse, which may be an advantage or a disadvantage, depending on the type of application on the enzyme-containing vesicles. One should also consider that the dehydration–rehydration cycle may inactivate the enzyme. It certainly depends on the type of applications and on the cost of the enzyme, whether achieving high encapsulation efficiencies is important or not. In the case of drug delivery, e.g., high EE values are usually desired.
From all these considerations on interactions of water-soluble enzymes with the vesicle membrane, it is clear that this aspect is important to be taken into account if one likes to understand the behavior of the entrapped enzymes. There are two main areas of applications of enzyme-containing lipid vesicles: (a) in the medical or biomedical field, particularly for the enzyme-replacement therapy, or (b) in the cheese ripening process (acceleration of the process and control over flavor development). For both of these types of applications, the lipid vesicles are just carriers for the enzymes, containers which protect the enzymes from getting in immediate contact with the medium to which they are added, the blood circulation. In the case of the enzyme-containing lipid vesicle-assisted cheese ripening process, the entrapped enzyme is gradually released, allowing catalyzing degradation and modification reactions in the cheese matrix during the ripening period. In the medical applications, the vesicles are used as the drug delivery system, the drug being the entrapped enzyme. In most cases, the vesicles carry enzyme molecules with the aim of replacing- or supporting-endogenous enzymes in the treatment of particular diseases (enzyme-replacement therapy). The entrapped enzyme molecules have to be released at a particular site in the body where they are needed [ 8 ].
Biodegradable polymers nanosystems are an attractive alternative to liposomes since they have the advantages of longer circulation in the blood stream and generally higher drug carrying capacity. Polymers such as poly(lactic acid) and poly(lactic-co-glycolic acid) have been extensively investigated for their biocompatibility and potential capability of releasing therapeutically proteins in a controlled way even over a prolonged period of time. These polymers are degradable by bulk erosion through hydrolysis of the ester bonds. The hydrolysis rate depends on several nanoparticles physicochemical parameters and can be tailored according to the desired release pattern of the protein to be incorporated. These two polymers were approved by FDA as excipients to achieve controlled release of the active ingredient. However, their application in protein delivery systems is often characterized by low entrapment efficiencies, burst release, instability of encapsulated hydrophilic protein, and partial protein release. To improve the performance of these polymer nanoparticles, polysaccharides such as alginate (ALG) and chitosan (CS) could be applied. CS and its derivatives have been intensively studied as carriers for proteins and drugs. More specifically these nanoparticles can be totally made by CS or used in several copolymer combinations. Copolymers made by the combination of CS/ALG are able to generate a more “friendly” environment which protects peptides and proteins from stressing conditions and allows their stabilization during encapsulation, storage, and release.
Kinetics properties of enzymes can be also altered during the entrapment process allowing potential change of the protein specificity to its substrate. Gaining a clear picture of these basics knowledge will definitely lead to a change of object design to increase the protein load, to control the protein release, and to retain the protein integrity and efficacy [ 9 ].
Bioaffinity interactions
Over the years, a number of protein–protein and protein–small molecule binding interactions have been harnessed for immobilization. These strategies exploit the selectivity of such interactions and are, therefore, highly specific with respect to the identity of binding partners as well as the location on the molecules at which binding occurs. Historically, many of the tags that have been described were developed for protein purification by affinity chromatography, but few have been widely co-opted for immobilization in other applications. Perhaps the most well-known genetically encoded affinity tag is the polyhistidine tag. This small tag, usually consisting of six sequential histidine residues, chelates transition metals including Cu(II), Co(II), Zn(II), or Ni(II), although the latter is most commonly employed. Here, a support bearing a chelating moiety such as nitrilotriacetic acid or imino-diacetic acid is treated with a solution of the relevant metal salt to produce a support presenting the metal ions. This metal-activated support is then used for protein immobilization through chelation with the histidine residues of the tag. This method of immobilization is widely used for the temporary capture of proteins during purification and is often termed immobilized metal affinity chromatography (IMAC) [ 7 ].
However, the general level of selectivity of this method is relatively low since several endogenous proteins have been identified that are also able to bind to metal ions, thus competing with the desired histidine-fused protein. As a result, for most applications the desired histidine-fusion protein must be purified prior to use. The strength of the binding interaction is also relatively weak ( K d ≈ 1–10 μM), although proteins bearing tags with 10–12 His residues or two separate histidine tags have been shown to give improvements of up to 1 order of magnitude, enabling in situ immobilization of the target protein on Ni-NTA slides directly from cell lysates [ 34 ].
Antibodies, apart from being the target of immobilization for use in microarrays and biosensors, may also be used as a means of immobilizing other proteins due to the selectivity of their binding interactions. This concept is regularly applied for purification through the use of columns with immobilized antibodies acting to trap their epitope target and is known as immunoaffinity chromatography. The method is, however, rarely used for other applications for several reasons. In order to achieve uniform immobilization, a well-defined monoclonal antibody is needed; polyclonal antibodies are unsuitable since they are not a single species but a heterogeneous population of antibodies that bind their epitope in a variety of conformations. Indeed, without in-depth structural knowledge of the binding interaction, it is impossible to determine strength of the binding or if the binding may block the active site of the protein of interest. Furthermore, the relatively weak nature of the binding means that it is often unsuitable for many applications that require longer-term or more robust immobilization of proteins.
Undoubtedly the most well-known and extensively researched protein-mediated immobilization technique relies on the non-covalent interaction of either avidin or streptavidin to proteins functionalized with biotin [ 35 ]. The interaction between biotin and (strept) avidin is extremely strong ( K d ≈ 10 −15 M), and this combined with the fact that these proteins are unusually stable to heat, denaturants, extremes of pH, and proteolysis means that the binding is essentially irreversible. The widespread availability of supports such as microtiter plates, microarray substrates, and magnetic particles that are coated with these proteins has also greatly contributed to the popularity of this method as a means of protein immobilization. However, in order to exploit this attachment for protein immobilization, the protein of interest must first be labeled with biotin. Classically, this can be achieved with a number of nonselective chemical biotinylation reagents such as biotin NHS ester [ 36 ].
One conceptually straightforward method of protein immobilization is through the use of enzymatically active fusion proteins. In this case, a protein of interest is fused to an enzyme (capture protein) that reacts selectively with an immobilized substrate analogue or inhibitor. This reaction forms a covalent bond between the enzyme and the substrate on the surface. This strategy was reported in 2002 by Mrksich, using the serine esterase cutinase from the filamentous fungus Fusarium solani pisi [ 37 ]. Cutinase is selectively inhibited by alkylphosphonate para -nitrophenol esters through esterification of the active site serine residue, resulting in the formation of a covalent bond. This protein and ligand were chosen because they possessed a number of desirable characteristics for an immobilization technique. The enzyme was relatively small (210 amino acid residues), globular, and monomeric, which would minimize any steric interactions with the fused protein. Both the termini are opposite to the active site and hence would be amenable to the generation of both N - and C -terminal fusions in which the fused protein would be oriented away from the support surface. Furthermore, the phosphonate diester inhibitor was relatively stable toward hydrolysis, and when bound in the cutinase active site, the alkyl tail of the phosphonate protruded out of the enzyme and offered an accessible location for attachment to the support.
There are also a number of other site-selective immobilization methods that do not require enzymatically mediated attachment. These rely on the recognition of specific functional groups present on amino acids in the protein or an associated peptide tag. One example of this employs phenolic oxidative cross-linking, a phenomenon that is widely observed in nature, which includes protein cross-linking through tyrosine residues [ 38 ]. Such dityrosine crosslinks occur in structural proteins such as elastin and silk and are catalyzed by metalloenzymes, although a simple complex of Ni(II) with the tripeptide glycine–glycine–histidine can also catalyze these reactions. Since IMAC and immobilization with Ni(II) via six-histidine tags and NTA-functionalized supports are already widely used, it was rationalized that this complex could be used as the cross-linking catalyst instead [ 39 ]. By bringing two tyrosine residues, one from the protein to be immobilized and another from the support, into close proximity to the Ni(II) complex, it would be possible to cross-link the two and form a covalent bond between the support and a specific location on the protein. Once the cross-linking had been achieved, the nickel was removed. The extent of dityrosine cross-linkage could also be easily determined since this moiety displays a characteristic fluorescence emission at 420 nm.
A wide range of biologically mediated methods are available for site-specific protein immobilization. This area of research is notable because it is the culmination of a major multidisciplinary research effort spanning molecular biology, protein engineering, organic chemistry, and materials science. The more recent introduction of biologically mediated methods for site-specific, particularly enzyme-mediated, immobilization of proteins from cell lysates should enable the future fabrication of more highly sensitive protein microarrays and biosensors as wells as finding new applications in nanotechnology and single molecule enzymology.
Support materials
The properties of supported enzyme preparations are governed by the properties of both the enzyme and the carrier material. The interaction between the two provides an immobilized enzyme with specific chemical, biochemical, mechanical, and kinetic properties. The support (carrier) can be a synthetic organic polymer, a biopolymer, or an inorganic solid [ 40 , 41 ]. A variety of matrixes have been used as support materials for enzyme immobilization [ 42 ].
Materials proposed in many examples are not suitable for industrial processing procedures due to their low mechanical strength [ 43 ]. This type of supporting matrixes is sometimes called soft gel.
Synthetic polymers
The most common and widely used synthetic polymers used as support for enzyme immobilization are represented by acrylic resins, such as Eupergit®-C (Evonik Industries). They are macroporous copolymers of N , N ′-methylene-bi-(methacrylamide), glycidyl methacrylate, allyl glycidyl ether, and methacrylamide with average particle size around 170 mm and a pore diameter of about 20–30 nm (Fig. 7 ). They are highly hydrophilic and stable, both chemically and mechanically, over a pH range from 0 to 14, and does not swell or shrink even upon drastic pH changes. A major drawback of hydrophilic resins is diffusion limitations, the effects of which, as would be expected, are more pronounced in kinetically controlled processes. Immobilization by covalent attachment to acrylic resins has been successfully applied to a variety of enzymes for industrial application [ 44 , 45 ].
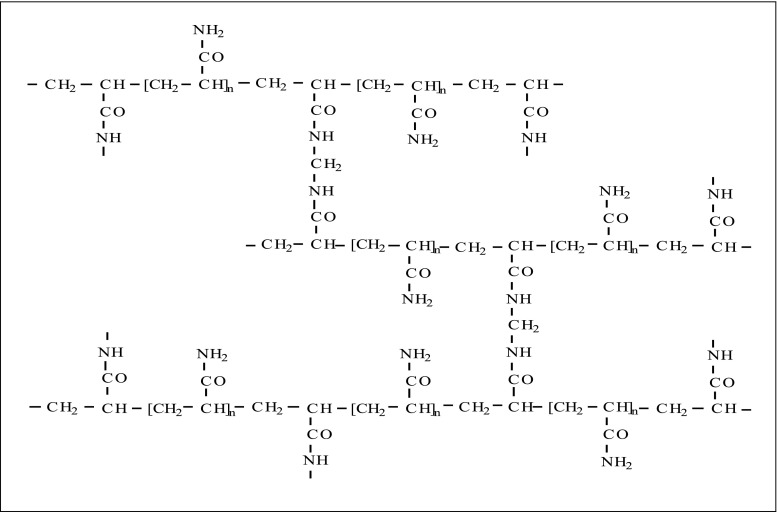
Structure of polyacrylamide gel matrix
As above reported, polyketone polymers –[–CO-CH 2 CH 2 –] n – appear promising supports for protein immobilization for their mechanical properties, high immobilization capacity, simplicity of the immobilization procedure by simple reactant mixing, and absence of bi-functional reagents or spacer arms. Polyketone polymers are obtained by palladium-catalyzed copolymerization of ethane and carbon monoxide [ 46 ]. Due to the close presence of polar keto groups, the polymers present a high liquid volume value (8.2 ml/g for a polymer not exceeding 25,000 Da), larger than twice than that shown by commercial sepharose, because the high possibility of hydrogen bonds between the keto groups and the water molecules and no restriction due to cross linking are present. Water accessible carbonyl groups were quantified in about 10 meq/g polymer, and the apparent concentration of the immobilized enzyme on the polyketone was more than 2.36 mg/ml [ 46 ].
Biopolymers
A variety of biopolymers, mainly water-insoluble polysaccharides such as cellulose, starch, agarose, carragenans, and chitosan, have been widely used as supports for immobilizing enzymes (Fig. 8 ). These matrixes form very inert aqueous gels, characterized by high gel strength, even at low concentration [ 47 ]. Moreover, due to their chemical structure, which can be easily activated, they may be prepared to bind proteins and enzymes in a reversible or irreversible way. Likewise, with the use of bi-functional reagents, it is possible to form cross-links which strengthen their structure increasing their mechanical and thermal resistance. Due to these particular characteristics, biopolymers are resins which are almost ideal to be used in purification (reversible binding) and in immobilization processes (irreversible binding) of biomolecules. Current techniques to conjugate polysaccharides to proteins include aldehyde, carbodiimide, epoxide, hydrazide, active ester, radical, and addition reactions, some of which are suitable for in situ conjugation of materials, while others are better suited for ex situ conjugation and purification.
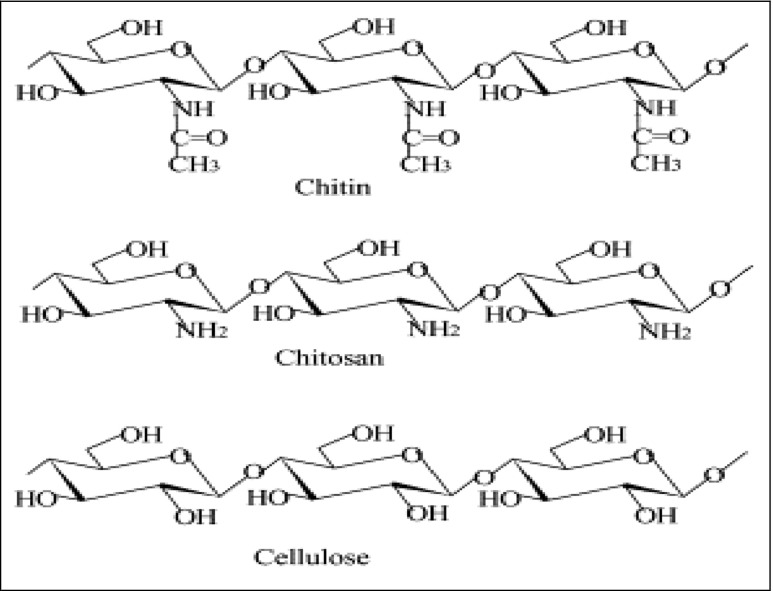
Chemical structures of cellulose and its amino derivatives
Enzymes can also be immobilized in natural or synthetic hydrogels or cryogels in non-aqueous media. Polyvinyl alcohol (PVA) cryogels formed by the freeze-thawing method, for example, have been widely used for immobilization of whole cells [ 48 ]. However, free enzymes, owing to their smaller size, can diffuse out of the gel matrix and are, consequently, leached in an aqueous medium. In order to entrap free enzymes, the size of the enzyme must be increased, e.g., by crosslinking. An alternative method to increase the size of the enzyme is to form a complex with a polyelectrolyte. Owing to their ampholytic character, proteins exist as polycations or polyanions, depending on the pH of the medium. Hence, they can often form complexes with oppositely charged polyelectrolytes.
Inorganic supports
On the other hand, rigid supports may be preferable. A variety of inorganic solids can be used for the immobilization of enzymes, e.g., alumina, silica, zeolites, and mesoporous silicas [ 49 ]. Silica-based supports are the most suitable matrices for enzyme immobilization in industrial manufacturing of enzyme-processed products [ 18 , 50 , 51 ], as well as for research purposes [ 52 ]. The most important advantages of silica gels compared to soft gels are their higher mechanical strength that allow their utilization for a much wider range of operating pressures and experimental conditions, as evidenced by their preferential use in the preparation of high performance liquid chromatography media [ 53 – 55 ]. Additionally, the surface of silica gel can be readily modified by chemical methods to provide various types of functional groups for facilitated ligand attachment. It has been shown that an extremely high loading of invertase was achieved by chemical modification of gel surface using amino group [ 56 ]. The overall surface area offered by silica gel matrixes is unparalleled. Furthermore, the silica gel can be easily fabricated to provide desirable morphology, pore structures, and micro-channels to allow substrate–ligand interaction. Furthermore, silica gel is mechanically stable and chemically inert, and it is therefore environmental- and solvent-friendly for industrial manufacturing and processing.
One of the simplest and most inexpensive methods to immobilize an enzyme on silica is by simple absorption. It is used, for example, to formulate enzymes for detergent powders which release the enzyme into the washing liquid during washing.
Smart polymers
A novel approach to immobilize enzymes is via covalent attachment to stimulus-responsive or mart polymer, which undergo dramatic conformational changes in response to small changes in their environment, e.g., temperature, pH, and ionic strength [ 57 ]. The most studied example is the thermo-responsive and biocompatible polymer, poly- N -isopropylacrylamide (polyNIPAM) [ 58 ]. Aqueous solutions of polyNIPAM exhibit a critical solution temperature (LCST) around 32 °C, below which the polymer readily dissolves in water while, above the LCST it becomes insoluble owing to expulsion of water molecules from the polymer network. Hence, the biotransformation can be performed under conditions where the enzyme is soluble, thereby minimizing diffusional limitations and loss of activity owing to protein conformational changes on the surface of a support. Subsequently, raising the temperature above the LCST leads to precipitation of the immobilized enzyme, thus facilitating its recovery and reuse. An additional advantage of using such thermo-responsive immobilized enzymes is that runaway conditions are avoided because when the reaction temperature exceeds the LCST, the catalyst precipitates and the reaction shuts down. Two methods are generally used to prepare the enzyme–polyNIPAM conjugates: (a) introduction of polymerizable vinyl groups into the enzyme followed by copolymerization with NIPAM or (b) reaction of NH 2 groups on the surface of the enzyme with a copolymer of NIPAM containing reactive ester groups or the homopolymer containing an N -succinimide ester function as the end group. More recently, an alternative thermo-responsive polymer has been described [ 59 – 61 ]. It consists of random copolymers derived from 2-(2-methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate and combines the positive features of poly(ethylene glycol), non-toxicity, and anti-immunogenicity, with thermo-responsive properties similar to polyNIPAM. The LCST could be varied between 26 and 90 °C depending on the relative amounts of OEGMA used. These properties make this a potentially interesting support for biocatalysts.
Conducting polymers
Polymers that exhibit electrical conductivity have now been successfully synthesized, and the past two decades have witnessed unending interest in the synthesis and characterization of such conducting polymers due to the potential technological applications of these materials. Conducting polymers also receive a great interest in biotechnology. A large number of organic compounds, which effectively transport charge, are roughly divided into three groups, i.e., charge transfer complexes/ion radical salts, organometallic species, and conjugated organic polymers. A new class of polymers known as intrinsically conducting polymers or electroactive conjugated polymers has recently emerged. Such materials exhibit interesting electrical and optical properties previously found only in inorganic systems. Electronically conducting polymers differ from all the familiar inorganic crystalline semiconductors, e.g., silicon in two important features that polymers are molecular in nature and lack long range order. A key requirement for a polymer to become intrinsically electrically conducting is that there should be an overlap of molecular orbitals to allow the formation of delocalized molecular wave function. Besides this, molecular orbitals must be partially filled so that there is a free movement of electrons throughout the lattice. Enzyme immobilization and biosensor construction are some of these applications [ 62 ].
Various conducting polymers have been considered for immobilization of enzymes. Of these, polypyrrole has gained most interest for the entrapment of protein molecules due to its low oxidation potential. This unique characteristic enables the growth of film from aqueous solutions that are compatible with most biological systems. This approach is usually based on entrapment of an enzyme into the structure of polypyrrole film by potentiostatic or galvanostatic polymerization of pyrrole in enzyme solution, which contains a supporting electrolyte.
Conducting polymers can be prepared by chemical or electrochemical polymerization. Although the chemical method offers mass production at a reasonable cost, the electrochemical method involves the direct formation of conducting polymer thin films with better control of thickness and morphology, which are more suitable for direct application. It is established that the conducting properties of the polymer films greatly depend on the mode of synthesis and also on the number of parameters, such as type of counter-ion, the type of electrolyte and its concentration, synthesis temperature, electrochemical voltage, pH of the electrolyte, etc. Thus, in order to improve the properties of polymer films suitable for a desired application, it is necessary to critically control and optimize the various synthesis parameters [ 63 ].
The other electrochemically prepared conducting polymers used for the biomolecule immobilization are polyacetylene, polythiophene, polyindole, and polyaniline. Also few biosensors based on insulating electropolymerized films polyphenol, poly( o -phenylenediamine), polydichlorophenolindophenol, and overoxidized polypyrrole have also been elaborated [ 64 ]. Many theoretical models have also been coupled with the electrochemical entrapment of enzyme for the evaluation of the role of the thickness of polymeric layers, enzyme location, enzyme loading, etc. on the biosensor functioning [ 65 ].
Gold nanoparticles
The progress of nanotechnology in the 1990s was followed by the rapid growth of nanobiotechnology. “Nanobiocatalysis” is one typical example. In the early approaches to nanobio-catalysis, enzymes were immobilized on various nanostructured materials using conventional approaches, such as simple adsorption and covalent attachment. This approach gathered attention by immobilizing enzymes onto a high surface area of nanostructured materials, such as nanoporous materials, electrospun nanofibers, and magnetic nanoparticles. This large surface area resulted in improved enzyme loading, which in turn increased the apparent enzyme activity per unit mass or volume compared to that of enzyme systems immobilized onto conventional materials. One of the particularly advantageous features of nanostructured materials is the control over size at the nanometer scale, such as the pore size in nanopores, thickness of nanofibers or nanotubes, and the particle size of nanoparticles. The uniform size distribution of nanomaterials and their similarity in size with enzyme molecules, together with other advantageous nanomaterial properties such as conductivity and magnetism, have revolutionized nanobiocatalytic approaches in various areas of enzyme technology and led to improved enzyme properties in nanobio-catalytic systems, particularly with regard to enzyme stability and activity. Recently, nanobiocatalytic approaches have evolved beyond simple enzyme immobilization strategies to include enzyme stabilization, wired enzymes, the use of enzymes in sensitive biomolecular detection, artificial enzymes, nanofabrication, and nanopatterning [ 66 ].
Nanosized particles of noble metals, especially gold nanoparticles (AuNPs), have received great interests due to their attractive electronic, optical, and thermal properties as well as catalytic properties and potential applications in the fields of physics, chemistry, biology, medicine, and material science. Therefore, the synthesis and characterization of AuNPs have attracted considerable attention from both a fundamental and a practical point of view. A variety of methods have been developed to prepare AuNPs [ 67 ]. However, the high surface energy of AuNPs makes them extremely reactive, and most systems undergo aggregation without protection or passivation of their surfaces. Thus, special precautions have to be taken to avoid their aggregation or precipitation. Typically, AuNPs are prepared by chemical reduction of the corresponding transition metal salts in the presence of a stabilizer which binds to their surface to impart high stability and a desirable linking chemistry and provide the desired charge and solubility properties. Some of the commonly used methods for surface passivation include protection by self-assembled monolayers, the most popular being citrate and thiol-functionalized organics; encapsulation in the H 2 O pools of reverse microemulsions; and dispersion in polymeric matrixes (Fig. 9 ). Although the synthesis of AuNPs makes great progress, the control of size, monodispersion, morphology, and surface chemistry of AuNPs is still a great challenge. Recently, designing novel protectors for AuNPs have been the focus of intense research because surface chemistry of AuNPs will play a key role in future application fields such as nanosensor, biosensor, catalysis, nanodevice, and nanoelectrochemistry.
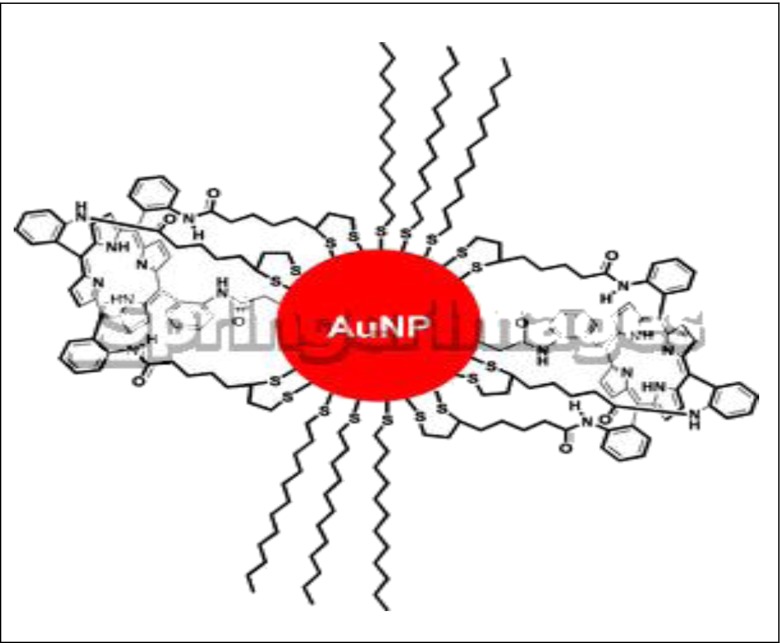
Porphyrin–gold nanoparticle (AuNP)
Magnetic nanoparticles
In recent years, substantial progress has been made in developing technologies in the field of magnetic carriers. Magnetic separation is an emerging technology that uses magnetism for the efficient separation of micro- and nanometer para- and ferro-magnetic particles from different chemical and biological samples.
With the rapid development of nanotechnology, magnetic nanoparticles are currently being widely studied. It has long been known that the physicochemical properties of magnetic nanoparticles can be vastly different from those of the corresponding bulk material. For example, magnetic anisotropy, which keeps a particle magnetized in a specific direction, is generally proportional to the volume of a particle [ 68 ]. Magnetic nanoparticles will display superparamagnetism, which means that the particles are attracted by a magnetic field, but retain no residual magnetism after the field is removed. Therefore, suspended superparamagnetic particles can be removed from solution using an external magnet, but they do not agglomerate (i.e., they stay suspended) after removal of the external magnetic field.
Among magnetic carriers, nano-sized magnetic particles possess many advantageous properties. Particles such as crosslinked iron oxide [ 69 , 70 ], ultrasmall superparamagnetic iron oxide [ 71 , 72 ], and monocrystalline iron oxide nano-particles [ 73 , 74 ] had all been developed as imaging agents in magnetic resonance imaging. Magnetic particles have been used to immobilize enzymes in order to enhance bioelement stability, ease separation from the reaction mixture, and increase catalyst stability, and they have been proposed for biotechnological applications [ 75 ] or for analytical devices, such as biosensors [ 76 , 77 ]. Furthermore, nanomedicine is an emerging field that uses nanoparticles to facilitate the diagnosis and treatment of diseases. Iron nanoparticles were used in tumor treatment relying on tumor vessel leakiness for preferential accumulation in cancer tissues. Specific targeting of magnetic nanoparticles to tumors has been accomplished in various experimental systems [ 78 ].
There have been various methods developed for the preparation of paramagnetic nanoparticles [ 79 ]. The most commonly used protocol involves co-precipitation of ferrous and ferric ions in basic solutions. The development of uniform nanometer sized particles has been intensively pursued because of their technological and fundamental scientific importance. These nanoparticular materials often exhibit very interesting electrical, optical, magnetic, and chemical properties, which cannot be achieved by their bulk counterparts. In literature, polymers such as dextran, PVA, and DEAE-starch were added to coat the particles for better stability, before or after the formation of iron oxide particles [ 80 , 81 ]. Otherwise, magnetic nanoparticles can be coated with silica containing a high coverage of silanol groups, which can easily be anchored with defined and generic surface chemistries [ 82 , 83 ]. As a result, magnetic nanoparticles coated with an ultrathin layer of silica would be more useful as magnetic nanocarrier [ 84 ].
Cross-linked enzyme aggregates (CLEAs)
Cross-linked enzyme crystals (CLECs) are highly active immobilized enzymes of controllable particle size, varying from 1 to 100 μm. Their operational stability and ease of recycling, coupled with their high catalyst and volumetric productivities, renders them ideally suited for industrial biotransformations. However, CLECs have an inherent disadvantage: Enzyme crystallization is a laborious procedure requiring enzyme of high purity, leading to their high costs. The more recently developed cross-linked enzyme aggregates (CLEAs), on the other hand, are produced by simple precipitation of the enzyme from aqueous solution, as physical aggregates of protein molecules, by the addition of salts, or water miscible organic solvents or non-ionic polymers [ 40 , 41 , 85 ]. These physical aggregates are held together by non-covalent bonding without perturbation of their tertiary structure that is without denaturation. Subsequent cross-linking of these physical aggregates renders them permanently insoluble while maintaining their pre-organized superstructure and hence their catalytic activity. This discovery led to the development of a new family of immobilized enzymes: CLEAs. This type of immobilized enzyme is very effective biocatalysts as they can be produced by inexpensive and effective method. CLEAs can readily be reused and exhibit satisfactory stability and performance for selected applications. The methodology is applicable to essentially any enzyme, including cofactor dependent oxidoreductases [ 40 , 41 ]. The use of CLEAs to penicillin acylase used in antibiotic synthesis has shown large improvements over other type of biocatalysts [ 86 ].
Selected applications of immobilized enzymes
Various applications of immobilized enzymes can be found in industry, medicine, and research. Table 1 has gathered a selection of currently used immobilized-enzyme processes. In any commercial application, the choice of a specific immobilized enzyme or mode of immobilization must be based on a specific compromise about all advantages and disadvantages between free and immobilized enzyme. Other industrial applications of immobilized enzymes include laboratory-scale organic synthesis and analytical and medical applications [ 87 , 88 ]. Furthermore, since enzymes are able to catalyze reactions not only in aqueous solutions but also in organic media, immobilized enzymes can catalyze organic synthesis [ 89 ].
Some important industrial applications of immobilized enzyme systems
In recent analytical applications, immobilized enzymes are used mainly in biosensors [ 90 ] and in diagnostic test strips. Biosensors are constructed by integrating biological sensing systems, e.g., enzyme (s), with a transducer. Enzymes for the most cases are immobilized either directly on a transducer’s working tip or using a polymer membrane tightly wrapping it up. In principle, due to enzyme specificity and sensitivity, biosensors can be tailored for nearly any target analyte [ 91 – 94 ], and these can be both enzyme substrates and enzyme inhibitors. Advantageously, their determination is performed without special preparation of the sample. The most extensively studied enzymes for the application in enzyme-based biosensors are presented in Table 2 . Practically, four of the sensors listed, namely glucose, lactate, urea, and glutamate, have been widely used and commercialized [ 90 ]. Recent advances were reviewed [ 95 – 97 ].
Some of the most frequently studied enzymes for enzyme-based biosensors
Enzyme biosensors are made by immobilization of enzymes on the sensing surface [ 98 – 100 ]. Due to the presence of reactive functional groups, organic polymeric carriers such as poly(2-glucosyloxyethyl methacrylate)-concanavalin [ 101 ], polyethylenimine [ 102 ], and polyvinylferrocenium [ 103 ] are used for this purpose. However, inorganic materials provide a better stability because of their thermal and mechanical stability and non-toxicity [ 104 ]. Various enzymes have been immobilized on inorganic materials, such as clay [ 105 ], porous silica, and alumina powder [ 106 ]. It is well-known that porous alumina membranes with various dimensions could be easily fabricated via electrical anodization of high purity aluminum. These membranes have been used to prepare nanoarrays and nanowires [ 107 – 109 ] and biosensors [ 110 – 112 ] .
Medical applications of immobilized enzymes include diagnosis and treatment of diseases. Offering a great potential in this area, real application of immobilized enzymes has as yet suffered from serious problems from their toxicity to the human organism, allergenic and immunological reactions, as well as from their limited stability in vivo. Examples of potential medical uses of immobilized enzyme systems are listed in Table 3 .
Selected potential medical uses of immobilized enzymes

Immobilization of digestive enzymes onto solid supports
The use of immobilized enzymes finds particular advantages using proteolytic enzymes. Papain, a thiol protease, present in the latex of Carica papaya exhibits a broad proteolytic activity and is an enzyme of industrial use and of high research interest. Papain has a variety of industrial applications, especially in food industry. It is used to tenderize meat and meat products, in the manufacture of protein hydrolysate, in brewing industry for clarifying juice and beer, in dairy industry for cheese, in backing industry, and in the extraction of flavor and color compounds from plants. Papain can also be used in forage industry to increase the utilization and transformation rate of proteins and in resolving plant and animal protein to make high health products [ 113 , 114 ]. The potential uses of papain include amino acid esters and peptide synthesis, treatment of acute destructive lactation mastitis, treatment of red blood cells prior to use in antibody-dependent cell-mediated cytotoxicity assays with lymphocytes, and enzyme inhibition-based biosensors for food safety and environmental monitoring [ 115 – 117 ].
Inorganic immobilization supports such as particulate aluminum oxides (alumina) have also been examined for immobilization of the proteolytic enzyme, papain [ 118 ]. In this research, organic phosphate linkers have been used for creation of free carboxyl groups in a two-step process. Papain binding to these alumina derivatives was performed using the water soluble carbodiimide 1-ethyl-3-(dimethylaminopropyl) carbodiimide. It has been shown that immobilized papain had similar kinetic constants compared to papain in solution. By fluorescence measurements, it was concluded that the hydrophobic environment of the active site remained unchanged, while the structure of the rest of the protein was perturbed by its association with the negatively charged surface [ 118 ].
Identification of proteins by liquid chromatography followed by mass spectrometry (LC–MS) is normally used as a suitable analytical method. In practice, proteins are subjected to proteolytic cleavage, and a complex mixture of peptides is produced. The peptide mixture is then separated by high-resolution LC–MS [ 119 – 121 ]. Protein digestion is normally performed in a homogeneous aqueous solution containing the proteolytic enzyme and the sample (enzyme-to-protein ratio, 1:50). However, this method suffers from several problems that may interfere with identification of sample proteins. Some of these problems include long digestion times (up to 24 h), auto-digestion by-products, and limited enzyme-to-substrate ratio.
Enzyme immobilization onto solid supports is a possible alternative to in-solution digestion. Different reactive groups of the supporting material (–OH, –NH 2 , and –COOH) can be utilized for covalent protein binding using relatively simple coupling strategies [ 122 ]. These approaches include co-polymerization with polyacrylamide gels [ 123 ], binding onto microbeads [ 124 ], silica-based substrates [ 125 , 126 ], synthetic polymers [ 127 ], and the inner walls of open capillaries or microchannels in microfluidics [ 128 , 129 ].
Textile industry
Another application of immobilized enzyme systems is found in textile industry where the main advantage is their low cost. The industrial plant size needed for continuous process is two orders of magnitude smaller than that required for batch process using free enzymes. The total costs are, therefore, considerably much lower. Immobilized enzymes offer greatly increased productivity on the basis of enzyme weight and also often provide process advantages [ 130 ].
Although oxidation reactions are essential in several industries, most of the conventional oxidation technologies have the following drawbacks: non-specific or undesirable side-reactions and use of environmentally hazardous chemicals. This has impelled the search for new oxidation technologies based on biological systems such as enzymatic oxidation. These systems show the following advantages over chemical oxidation: Enzymes are specific and biodegradable catalysts and enzyme reactions are carried out under mild conditions.
Enzymatic oxidation techniques have been proposed in a great variety of industrial fields including pulp and paper, textile, and food industries. Recycling oxidizing enzymes on molecular oxygen as electron acceptor are the most interesting ones. Thus, laccases (benzenediol: oxygen oxidoreductase; EC 1.10.3.2) belong to a particularly promising class of enzymes for the above-mentioned purposes [ 131 ]. The laccase molecule is a dimeric or tetrameric glycoprotein, which usually contains four copper atoms per monomer distributed in three redox sites. This enzyme catalyzes the oxidation of ortho- and paradiphenols, aminophenols, polyphenols, polyamines, lignins, and aryl diamines as well as some inorganic ions coupled to the reduction of molecular dioxygen to water [ 132 ].
A wide variety of supports and methods are constantly used for the immobilization of various industrial enzymes, and several applications of laccases have been proposed. Specifically, the direct substrate oxidation of phenol derivatives has been investigated in bioremediation efforts to decontaminate industrial wastewaters. The polymeric polyphenolic derivatives that result from the laccase-catalyzed oxidative couplings are usually insoluble and can be separated, easily, by filtration or sedimentation Additionally, a laccase has been commercialized, recently, for preparing cork stoppers for wine bottles, whereby the enzyme oxidatively diminishes the characteristic cork taint and/or astringency that is frequently imparted to aged bottled wine [ 133 ].
At present, the main technological applications of laccases are in the textile, in processes related to decolorization of dyes and in the pulp and paper industries for the delignification of woody fibers, particularly during the bleaching process [ 134 ]. In most of these applications, laccases are used together with a chemical mediator [ 135 ]. Currently, all marketed laccases are of fungal origin, but the recent identification and structure determination of a bacterial laccase may eventually broaden the horizon for this enzyme class. It remains to be seen whether bacterial enzymes can be expressed at levels sufficient for their commercialization.
Conclusions
The technology of immobilized enzymes is still going through a phase of evolution and maturation. Evolution is reflected in the ever-broadening range of applications of immobilized enzymes. Maturation is mirrored in the development of the theory of how immobilized enzymes function and how the technique of immobilization is related to their primary structure through the formation and configuration of their three dimensional structure. There still remains much room for the development of useful processes and materials based on this hard-won understanding. Immobilized enzymes will clearly be more widely used in the future. This is just the beginning of the immobilized enzyme technology era.
Acknowledgments
We would like to thank the financial support by Hormozgan Science and Technology Park.
Abbreviations
Cross-linked enzyme aggregates
Cross-linked enzyme crystals
Cross-linked iron oxide
Dimethylaminoethyl
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide
Food and Drug Association
High-performance liquid chromatography
Immobilized metal affinity chromatography
Dissociation constant
Michaelis constant
Liquid chromatography–mass spectrometry
Low critical solution temperature
Monocrystalline iron oxide nanoparticles
N -Hydroxysuccinimide
Nickel nitrilotriacetic acid
Poly(lactic acid)
Poly(lactic-co-glycolic acid)
Poly- N -isopropylacrylamide
Polyvinyl alcohol
Transition temperature
Ultrasmall superparamagnetic iron oxide
Contributor Information
Ahmad Abolpour Homaei, Email: [email protected].
Reyhaneh Sariri, Email: [email protected].
Roberto Stevanato, Email: [email protected].
- 1. Gomes-Ruffi CR, Henrique da Cunha R, Almeida EL, Chang YK, Steel KJ. Effect of the emulsifier sodium stearoyl lactylate and of the enzyme maltogenic amylase on the quality of pan bread during storage. LWT- Food Sci Technol. 2012;49(1):96–101. [ Google Scholar ]
- 2. Jaros D, Rohm H (2011) Enzymes Exogenous to Milk in Dairy Technology Transglutaminase Encyclopedia of Dairy Sciences (Second Edition) 297–300
- 3. Ismail B, Nielsen SS. Invited review: plasmin protease in milk: current knowledge and relevance to dairy industry. J Dairy Sci. 2010;93(11):4999–5009. doi: 10.3168/jds.2010-3122. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Bai Y, Huang H, Meng K, Shi P, Yang P, Luo H, Luo C, Feng Y, Zhang W, Yao B. Identification of an acidic α-amylase from Alicyclobacillus sp. A4 and assessment of its application in the starch industry. Food Chem. 2012;113(4):1473–1478. [ Google Scholar ]
- 5. Schückel J, Matura A, van Pée KH. One-copper laccase-related enzyme from Marasmius sp.: purification, characterization and bleaching of textile dyes. Enzyme Microb Technol. 2011;48(3):278–284. doi: 10.1016/j.enzmictec.2010.12.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Hakala TK, Liitiä T, Suurnäkki A. Enzyme-aided alkaline extraction of oligosaccharides and polymeric xylan from hardwood kraft pulp. Carbohydr Polym. 2013;93(1):102–108. doi: 10.1016/j.carbpol.2012.05.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Rao CS, Sathish T, Ravichandra P, Prakasham RS. Characterization of thermo- and detergent stable serine protease from isolated Bacillus circulans and evaluation of eco-friendly applications. Process Biochem. 2009;44(3):262–268. [ Google Scholar ]
- 8. Soldatkin OO, Kucherenko IS, Pyeshkova VM, Kukla AL, Jaffrezic-Renault N, El'skaya AV, Dzyadevych SV, Soldatkin AP. Novel conductometric biosensor based on three-enzyme system for selective determination of heavy metal ions. Bioelectrochemistry. 2012;83:25–30. doi: 10.1016/j.bioelechem.2011.08.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Apetrei IM, Rodriguez-Mendez ML, Apetrei C, de Saja JA. Enzyme sensor based on carbon nanotubes/cobalt(II) phthalocyanine and tyrosinase used in pharmaceutical analysis. Sens Actuators B. 2013;177:138–144. [ Google Scholar ]
- 10. Das R, Ghosh S, Bhattacharje C. Enzyme membrane reactor in isolation of antioxidative peptides from oil industry waste: a comparison with non-peptidic antioxidants. LWT- Food Sci Technol. 2012;47(2):238–245. [ Google Scholar ]
- 11. Atadashi IM, Aroua MK, Abdul Aziz A. High quality biodiesel and its diesel engine application: a review. Renew Sustain Energy Rev. 2010;14:1999–2008. [ Google Scholar ]
- 12. Luo K, Yang Q, Yu J, Li XM, Yang GJ, Xie BX, Yang F, Zheng W, Zeng GM. Combined effect of sodium dodecyl sulfate and enzyme on waste activated sludge hydrolysis and acidification. Bioresour Technol. 2011;102(14):7103–7110. doi: 10.1016/j.biortech.2011.04.023. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Tonini D, Astrup T. Life-cycle assessment of a waste refinery process for enzymatic treatment of municipal solid waste. Waste Manag. 2012;32(1):165–176. doi: 10.1016/j.wasman.2011.07.027. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Tong Z, Qingxiang Z, Hui H, Qin L, Yi Z (1997) Removal of toxic phenol and 4-chlorophenol from waste water by horseradish peroxidase 34(4):893–903
- 15. Gupta A, Khare SK. Enzymes from solvent-tolerant microbes: useful biocatalysts for non-aqueous enzymology. Crit Rev Biotechnol. 2009;29:44–54. doi: 10.1080/07388550802688797. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Hartmann M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem Mater. 2005;17:4577–4593. [ Google Scholar ]
- 17. Kallenberg AI, van Rantwijk F, Sheldon RA. Immobilization of penicillin G acylase: the key to optimum performance. Adv Synth Catal. 2005;347:905–926. [ Google Scholar ]
- 18. Pierre AC. The sol–gel encapsulation of enzymes. Biocatal Biotransfor. 2004;22:145–170. [ Google Scholar ]
- 19. Cherry JR, Fidantsef AL. Directed evolution of industrial enzymes: an update. Curr Opin Biotechnol. 2003;14:438–443. doi: 10.1016/s0958-1669(03)00099-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Kress J, Zanaletti R, Amour A, Ladlow M, Frey JG, Bradley M. Enzyme accessibility and solid supports: which molecular weight enzymes can be used on solid supports? An investigation using confocal Raman microscopy. Chem Eur J. 2002;8:3769–3772. doi: 10.1002/1521-3765(20020816)8:16<3769::AID-CHEM3769>3.0.CO;2-V. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Bommarius AS, Riebel BR (2004) Biocatalysis: fundamentals and applications. Wiley-VCH, Weinheim
- 22. Massolini G, Calleri E. Immobilized trypsin systems coupled on-line to separation methods: recent developments and analytical applications. J Sep Sci. 2005;28:7–21. doi: 10.1002/jssc.200401941. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Cao L, van Langen L, Sheldon RA. Immobilised enzymes: carrier-bound of carrier-free? Curr Opin Biotechnol. 2003;14:387–394. doi: 10.1016/s0958-1669(03)00096-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CT-W, Olson DH, Sheppard EW, McCullen SB, Higgins JB, Schlenkert JL. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc. 1992;114:10834–10843. [ Google Scholar ]
- 25. Deere J, Magner E, Wall JG, Hodnett BK. Mechanistic and structural features of protein adsorption onto mesoporous silicates. J Phys Chem B. 2002;106:7340–7347. [ Google Scholar ]
- 26. Cang-Rong JT, Pastorin G. The influence of carbon nanotubes on enzyme activity and structure: investigation of different immobilization procedures through enzyme kinetics and circular dichroism studies. Nanotechnology. 2009;20:1–20. doi: 10.1088/0957-4484/20/25/255102. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Köhn M, Breinbauer R. The Staudinger ligation—a gift to chemical biology. Angew Chem. 2004;43:3106–3116. doi: 10.1002/anie.200401744. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Soellner MB, Dickson KA, Nilsson BL, Raines RT. Site-specific protein immobilization by Staudinger ligation. J Am Chem Soc. 2003;125:11790–11791. doi: 10.1021/ja036712h. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Kalia J, Abbott NL, Raines RT. General method for site-specific protein immobilization by Staudinger ligation. Bioconjug Chem. 2007;18:1064–1069. doi: 10.1021/bc0603034. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Kalia J, Raines RT. Reactivity of intein thioesters: appending a functional group to a protein. Chembiochem. 2006;7:1375–1383. doi: 10.1002/cbic.200600150. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Moses JE, Moorhouse AD. The growing applications of click chemistry. Chem Soc Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA. Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med Res Rev. 2008;28:278–308. doi: 10.1002/med.20107. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Song W, Wang Y, Qu J, Lin Q. Selective functionalization of a genetically encoded alkene-containing protein via “photoclick chemistry” in bacterial cells. J Am Chem Soc. 2008;130:9654–9655. doi: 10.1021/ja803598e. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Lauer SA, Nolan JP. Development and characterization of Ni-NTA-bearing microspheres. Cytometry. 2002;48:136–145. doi: 10.1002/cyto.10124. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Hutsell SQ, Kimple RJ, Siderovski DP, Willard FS, Kimple AJ. High-affinity immobilization of proteins using biotin- and GST-based coupling strategies. Methods Mol Biol. 2010;627:75–90. doi: 10.1007/978-1-60761-670-2_4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Chattopadhaya S, Abu Bakar FB, Yao SQ. Expanding the chemical biologist’s tool kit: chemical labelling strategies and its applications. Curr Med Chem. 2009;16:4527–4543. doi: 10.2174/092986709789760706. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Hodneland CD, Lee YS, Min DH, Mrksich M. Selective immobilization of proteins to self-assembled monolayers presenting active site-directed capture ligands. Proc Natl Acad Sci. 2002;99:5048–5052. doi: 10.1073/pnas.072685299. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Stayner RS, Min DJ, Kiser PF, Stewart RJ. Site-specific cross-linking of proteins through tyrosine hexahistidine tags. Bioconjug Chem. 2005;16:1617–1623. doi: 10.1021/bc050249b. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Endrizzi BJ, Huang G, Kiser PF, Stewart RJ. Specific covalent immobilization of proteins through dityrosine cross-links. Langmuir. 2006;22:11305–11310. doi: 10.1021/la0618216. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Sheldon RA. Enzyme immobilization: the quest for optimum performance. Adv Synth Catal. 2007;349:1289–1307. [ Google Scholar ]
- 41. Sheldon RA. Cross-linked enzyme aggregates (CLEAs): stable and recyclable biocatalysts. Biochem Soc Trans. 2007;35:1583–1587. doi: 10.1042/BST0351583. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Girelli AM, Mattei E. Application of immobilized enzyme reactor in on-line high performance liquid chromatography: a review. J Chromatogr B. 2005;819:3–16. doi: 10.1016/j.jchromb.2005.01.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Sakaguchi K, Matsui M, Mizukami F. Applications of zeolite inorganic composites in biotechnology: current state and perspectives. Appl Microbiol Biotechnol. 2005;67:306–311. doi: 10.1007/s00253-004-1782-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Boller T, Meier C, Menzler S. EUPERGIT oxirane acrylic beads: how to make enzymes fit for biocatalysis. Org Process Res Dev. 2002;6:509–519. [ Google Scholar ]
- 45. Katchalski-Katzir E, Kraemer DM. Eupergit® C, a carrier for immobilization of enzymes of industrial potential. J Mol Catal B Enzym. 2000;10:157–176. [ Google Scholar ]
- 46. Agostinelli E, Belli F, Tempera G, Mura A, Floris G, Toniolo L, Vavasori A, Fabris, Momo F, Stevanato R. Polyketone polymer: a new support for direct enzyme immobilization. J Biotechnol. 2007;127:670–678. doi: 10.1016/j.jbiotec.2006.08.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. van de Velde F, Lourenço ND, Pinheiro HM, Bakker M. Carrageenan: a food-grade and biocompatible support for immobilisation techniques. Adv Synth Catal. 2002;344:815–835. [ Google Scholar ]
- 48. Tripathi A, Sami H, Jain SR, Viloria-Cols M, Zhuravleva N, Nilsson G, Jungvid H, Kumar A. Improved bio-catalytic conversion by novel immobilization process using cryogel beads to increase solvent production. Enzym Microb Technol. 2010;47:44–51. [ Google Scholar ]
- 49. Hudson S, Cooney J, Magner E. Proteins in mesoporous silicates. Angew Chem. 2008;47:8582–8594. doi: 10.1002/anie.200705238. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Blanco RM, Terreros P, Fernandez-Perez M, Otero C, Diaz-Gonzalez G. Fictionalization of mesoporous silica for lipase immobilization—characterization of the support and the catalysts. J Mol Catal B Enzym. 2004;30:83–93. [ Google Scholar ]
- 51. Ho LF, Li SY, Lin SC, Wen-Hwei H. Integrated enzyme purification and immobilization processes with immobilized metal affinity adsorbents. Process Biochem. 2004;39:1573–1581. [ Google Scholar ]
- 52. Vianello F, Zennaro L, Di Paolo ML, Rigo A, Malacarne C, Scarpa M. Preparation, morphological characterization and activity of thin films of horseradish peroxidase. Biotech Bioeng. 2000;68:488–495. [ PubMed ] [ Google Scholar ]
- 53. Cabrera K. Applications of silica-based monolithic HPLC columns. J Sep Sci. 2004;27:843–852. doi: 10.1002/jssc.200401827. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Majors RE. Developments in HPLC column packing design. LCGC. 2006;24:8–15. [ Google Scholar ]
- 55. Xu L, Feng YQ, Da SL, Shi ZG. Applications of ordered mesoporous materials in separation science. Chin J Anal Chem. 2004;32:374–380. [ Google Scholar ]
- 56. David AE, Wang NS, Yang VC, Yang AJ. Chemically surface modified gel (CSMG): an excellent enzyme-immobilization matrix for industrial processes. J Biotechnol. 2006;125:395–407. doi: 10.1016/j.jbiotec.2006.03.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Klis M, Karbarz M, Stojek Z, Rogalski J, Bilewicz R. Thermoresponsive poly(N-isopropylacrylamide) gel for immobilization of laccase on indium tin oxide electrodes. J Phys Chem B. 2009;113:6062–6067. doi: 10.1021/jp8094159. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Lozinsky VI, Simenel IA, Kulakova VK, Kurskaya EA, Babushkina TA, Klimova TP, Burova TV, Dubovik AS, Grinberg VY, Galaev IY, Mattiasson B, Khokhlov AR. Synthesis and studies of N-vinylcaprolactam/N-vinylimidazole copolymers that exhibit the “protein-like” behaviour in aqueous media. Macromolecules. 2003;36:7308–7323. [ Google Scholar ]
- 60. Virtanen J, Tenhu H. Thermal properties of poly(N-isopropylacrylamide)-g-poly(ethyleneoxide) in aqueous solutions: influence of the number and distribution of the grafts. Macromolecules. 2000;33:5970–5975. [ Google Scholar ]
- 61. Virtanen J, Baron C, Tenhu H. Grafting of poly(N-isopropylacrylamide) with poly(ethyleneoxide) under various reaction conditions. Macromolecules. 2000;33:336–341. [ Google Scholar ]
- 62. Cirpan A, Alkan S, Toppare L, Hepuzer Y, Yagci Y. Immobilization of invertase in conducting copolymers of 3-methylthienyl methacrylate. Bioelectrochemistry. 2003;59:29–33. doi: 10.1016/s1567-5394(02)00186-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Kiralpa S, Balika B, Karatasb S, Toppare L, Gungor A. An alternative supporting electrolyte for enzyme immobilization in conducting polymers. Int J Biol Macromol. 2008;42:191–194. doi: 10.1016/j.ijbiomac.2007.09.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Lange U, Roznyatovskaya NV, Mirsky VM. Conducting polymers in chemical sensors and arrays. Anal Chim Acta. 2008;614:1–26. doi: 10.1016/j.aca.2008.02.068. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Gerard M, Chaubey A, Malhotra BD. Application of conducting polymers to biosensors. Biosens Bioelectron. 2002;17:345–359. doi: 10.1016/s0956-5663(01)00312-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Chow DC, Johannes MS, Lee W-K, Clark RL, Zauscher S, Chilkoti A. Nanofabrication with biomolecules. Mater Today. 2005;8:30–39. [ Google Scholar ]
- 67. Daniel M-C, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Selvan ST, Hayakawa T, Nogami M, Kobayashi Y, Liz-Marzan LM, Hamanaka Y, Nakamura AJ. Sol–gel derived gold nanoclusters in silica glass possessing large optical nonlinearities. Phys Chem B. 2002;106:10157–10162. [ Google Scholar ]
- 69. Schellenberger EA, Bogdanov AJ, Hogemann D, Tait J, Weissleder R, Josephson L. Annexin V-CLIO: a nanoparticle for detecting apoptosis by MRI. Mol Imaging. 2002;2:102–107. doi: 10.1162/15353500200202103. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Wunderbaldinger P, Josephson L, Weissleder R. Crosslinked iron oxides (CLIO): a new platform for the development of targeted MR contrast agents. Acad Radiol. 2002;9:S304–S306. doi: 10.1016/s1076-6332(03)80210-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Keller TM, Michel SC, Frohlich J, Fink D, Caduff R, Marincek B, Kubik-Huch RA. USPIO-enhanced MRI for preoperative staging of gynecological pelvic tumors: preliminary results. Eur Radiol. 2004;14:937–944. doi: 10.1007/s00330-004-2258-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Funovics MA, Kapeller B, Hoeller C, Su HS, Kunstfeld R, Puig S, Macfelda K. MR imaging of the her2/neu and 9.2.27 tumor antigens using immunospecific contrast agents. Magn Reson Imaging. 2004;22:843–850. doi: 10.1016/j.mri.2004.01.050. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Krause MH, Kwong KK, Gragoudas ES, Young LH. MRI of blood volume with superparamagnetic iron in choroidal melanoma treated with thermotherapy. Magn Reson Imaging. 2004;22:779–787. doi: 10.1016/j.mri.2004.01.052. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Kluchova K, Zboril R, Tucek J, Pecova M, Zajoncova L, Safarik I, Mashlan M, Markova I, Jancik D, Sebela M, Bartonkova H, Bellesi V, Novak P, Petridis D. Superparamagnetic maghemite nanoparticles from solid-state synthesis—their functionalization towards peroral MRI contrast agent and magnetic carrier for trypsin immobilization. Biomaterials. 2009;30:2855–63. doi: 10.1016/j.biomaterials.2009.02.023. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Kouassi G, Irudayaraj J, McCarty G. Activity of glucose oxidase functionalized onto magnetic nanoparticles. Biomagn Res Technol. 2005;3:1–10. doi: 10.1186/1477-044X-3-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Tsang SC, Yu CH, Gao X, Tam K. Silica-encapsulated nanomagnetic particles as a new recoverable biocatalyst carrier. J Phys Chem B. 2006;110:16914–16922. doi: 10.1021/jp062275s. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, Derfus AM, Yang M, Hoffman RM, Bhatia S, Sailor MJ, Ruoslahti E. Biomimetic amplification of nanoparticle homing to tumors. Proc Anal Nat Sci. 2007;104:932–936. doi: 10.1073/pnas.0610298104. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem. 2004;14:2161–2175. [ Google Scholar ]
- 80. Bergemann C, Muller-Schulte D, Oster J, Brassard LA, Lubbe AS. Magnetic ion-exchange nano- and microparticles for medical biochemical and molecular biological applications. J Magn Magn Mater. 1999;194:45–52. [ Google Scholar ]
- 81. Lee J, Isobe T, Senna M. Preparation of ultrafine Fe3O4 particles by precipitation in the presence of PVA at high pH. J Colloid Interface Sci. 1996;177:490–494. [ Google Scholar ]
- 82. Laurent N, Haddoub R, Flitsch SL. Enzyme catalysis on solid surfaces. Trends Biotechnol. 2008;26:328–37. doi: 10.1016/j.tibtech.2008.03.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterization and biochemical applications. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Li Y, Yan B, Deng C, Yu W, Xu X, Yang P, Zhang X. Efficient on-chip proteolysis system based on functionalized magnetic silica microspheres. Proteomics. 2007;7:2330–2339. doi: 10.1002/pmic.200700112. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Sheldon RA, Schoevaart R, van Langen L. A novel method for enzyme immobilization. Biocat Biotrans. 2005;2005:141–147. [ Google Scholar ]
- 86. Illanes A, Wilson L, Caballero E, Fernandez-Lafuente R, Guisan JM. Crosslinked penicillin acylase aggregates for synthesis of beta-lactam antibiotics in organic medium. Appl Biochem Biotechnol. 2006;133:189–202. doi: 10.1385/abab:133:3:189. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Brena BM, Batista-Viera F (2006) Immobilization of enzymes: a literature survey. In: Guisan JM (ed) Immobilization of enzymes and cells, 2nd edn. Springer, New York, pp 15–30
- 88. Taylor IN, Brown RC, Bycroft M, King G, Littlechild JA, Lloyd MC, Praquin C, Toogood HS, Taylor SJ. Application of thermophilic enzymes in commercial biotransformation processes. Biochem Soc Trans. 2004;32:290–292. doi: 10.1042/bst0320290. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Carrea G, Riva S. Properties and synthetic applications of enzymes in organic solvents. Angew Chem. 2000;39:2226–2254. [ PubMed ] [ Google Scholar ]
- 90. Wilson GS, Hu Y. Enzyme-based biosensors for in vivo measurements. Chem Rev. 2000;100:2693–2704. doi: 10.1021/cr990003y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Vianello F, Bortoluzzi S, Zennaro L, Rigo A. Determination of glucose oxidase immobilised as monolayer onto a flat surface. J Biochem Biophys Methods. 2002;51:263–271. doi: 10.1016/s0165-022x(02)00027-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Vianello F, Cambria A, Ragusa S, Cambria MT, Rigo A. A high sensitivity amperometric biosensor using a monomolecular layer of laccase as biorecognition element. Biosens Bioelectron. 2004;20:315–321. doi: 10.1016/j.bios.2004.01.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Vianello F, Ragusa S, Cambria MT, Rigo A. A high sensitivity amperometric biosensor using laccase as biorecognition element. Biosens Bioelectron. 2006;21:2155–2160. doi: 10.1016/j.bios.2005.10.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Vianello F, Zennaro L, Rigo A. A coulometric biosensor to determine hydrogen peroxide using a monomolecular layer of horseradish peroxidase immobilized on a glass surface. Biosens Bioelectron. 2007;22:2694–2699. doi: 10.1016/j.bios.2006.11.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Chudy M, Grabowska I, Ciosek P, Filipowicz-Szymanska A, Stadnik D, Wyzkiewicz I, Jedrych E, Juchniewicz M, Skolimowski M, Ziolkowska K, Kwapiszewski R. Miniaturized tools and devices for bioanalytical applications: an overview. Anal Bioanal Chem. 2009;395:647–668. doi: 10.1007/s00216-009-2979-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Rapp BE, Gruhl FJ, Länge K (2010) Biosensors with label-free detection designed for diagnostic applications. Anal Bioanal Chem 398:2403–2412 [ DOI ] [ PubMed ]
- 97. Ronkainen NJ, Halsall HB, Heineman WR. Electrochemical biosensors. Chem Soc Rev. 2010;39:1747–1763. doi: 10.1039/b714449k. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Jin W, Brennan JD. Properties and applications of proteins encapsulated within sol–gel derived materials. Anal Chim Acta. 2002;461:1–36. [ Google Scholar ]
- 99. Lee J, Kim J, Kim J, Jia H, Kim MI, Kwak JH, Jin S, Dohnalkova A, Park HG, Chang HN, Wang P, Grate JW, Hyeon T. Simple synthesis of hierarchically ordered mesocellular mesoporous silica materials hosting crosslinked enzyme aggregates. Small. 2005;1:744–753. doi: 10.1002/smll.200500035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Vinu A, Murugesan V, Tangermann O, Hartmann M. Adsorption of cytochrome c on mesoporous molecular sieves: influence of pH, pore diameter, and aluminum incorporation. Chem Mater. 2004;16:3056–3065. [ Google Scholar ]
- 101. Miyata T, Jikihara A, Nakamae K, Hoffman AS. Preparation of reversibly glucose-responsive hydrogels by covalent immobilization of lectin in polymer networks having pendant glucose. J Biomater Sci Polym. 2004;15:1085–1098. doi: 10.1163/1568562041753061. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Lakard B, Herlem G, Lakard S, Antoniou A, Fahys B. Urea potentiometric biosensor based on modified electrodes with urease immobilized on polyethylenimine films. Biosens Bioelectron. 2004;19:1641–1646. doi: 10.1016/j.bios.2003.12.035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Kuralay F, Özyörük KH, Yıldız A. Potentiometric enzyme electrode for urea determination using immobilized urease in poly(vinylferrocenium) film. Sens Actuators B. 2005;109:194–199. [ Google Scholar ]
- 104. Tischer W, Wedekind F. Immobilized enzymes: methods and applications. Top Curr Chem. 1999;200:95–126. [ Google Scholar ]
- 105. Sanjay G, Sugunan S. Glucoamylase immobilized on montmorillonite: synthesis, characterization & starch hydrolysis activity in a fixed bed reactor. Catal Commun. 2005;6:525–529. [ Google Scholar ]
- 106. Reshmi R, Sanjay G, Sugunan S. Enhanced activity and stability of a-amylase immobilized on alumina. Catal Commun. 2006;7:460–465. [ Google Scholar ]
- 107. Martin CR. Nanomaterials: a membrane-based synthetic approach. Science. 1994;266:1961–1966. doi: 10.1126/science.266.5193.1961. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Metzger R, Konovalov V, Sun M, Xu T, Zangari G, Xu B, Benakli M. Magnetic nanowires in hexagonally ordered pores of alumina. IEEE Trans Magn. 2000;36:30–35. [ Google Scholar ]
- 109. Xiao Z, Han C, Welp U, Wang H, Kwok W, Willing G, Hiller J, Cook R, Miller D, Crabtree G. Fabrication of alumina nanotubes and nanowires by etching porous alumina membranes. Nano Lett. 2002;2:1293–1297. [ Google Scholar ]
- 110. Kohli P, Wirtz M, Martin CR. Nanotube membrane based biosensors. Electroanalysis. 2004;16:9–12. [ Google Scholar ]
- 111. Steinle ED, Mitchell DT, Wirtz M, Lee SB, Young VY, Martin CR. Ion channel mimetic micropore and nanotube membrane sensors. Anal Chem. 2002;74:2416–2421. doi: 10.1021/ac020024j. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Vlassiouk PT, Smirnov S. Sensing DNA hybridization via ionic conductance through a nanoporous electrode. Langmuir. 2005;21:4776–4778. doi: 10.1021/la0471644. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Monti R, Basilio CA, Trevisan HC, Contiero J. Purification of papain from fresh latex of Carica papaya. Braz Arch Biol Technol. 2000;43:501–507. [ Google Scholar ]
- 114. Sumantha A, Larroche C, Pandey A. Microbiology and industrial biotechnology of food-grade proteases: a perspective. Food Technol Biotechnol. 2006;44:211–20. [ Google Scholar ]
- 115. Afaq S, Iqbal J. Immobilization and stabilization of papain on chelating sepharose: a metal chelate regenerable carrier. Electron J Biotechnol. 2001;4:120–124. [ Google Scholar ]
- 116. Homaei A, Sajedi R, Seifzadeh S, Sariri R, Stevanato R. Cystein enhances activity and stability of immobilized papain. Amino Acids. 2010;38:937–942. doi: 10.1007/s00726-009-0302-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Shukor Y, Baharom NA, Abd Rahman F, Puad Abdullah M, Shamaan NA, Arif Syed M. Development of a heavy metals enzymatic-based assay using papain. Anal Chim Acta. 2006;566:283–288. [ Google Scholar ]
- 118. Hyndman D, Burrell R, Lever G, Flynn TG. Protein immobilization to alumina supports: II. Papain immobilization to alumina via organophosphate linkers. Biotechnol Bioeng. 2004;40:1328–1336. doi: 10.1002/bit.260401106. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Han X, Aslanian A, Yates JR. Mass spectrometry for proteomics. Curr Opin Chem Biol. 2008;12:483–487. doi: 10.1016/j.cbpa.2008.07.024. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 120. Kalli A, Hakansson K. Comparison of the electron capture dissociation fragmentation behavior of doubly and triply protonated peptides from trypsin, Glu-C, and chymotrypsin digestion. J Proteome Res. 2008;7:2834–2838. doi: 10.1021/pr800038y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Tolmachev AV, Monroe ME, Purvine SO, Moore RJ, Jaitly N, Adkins JN, Anderson GA, Smith RD. Characterization of strategies for obtaining confident identifications in bottom-up proteomics measurements using hybrid FTMS instruments. Anal Chem. 2008;80:8514–8519. doi: 10.1021/ac801376g. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 122. Bryjak J, Kruczkiewicz P, Rekuć A, Peczyńska-Czoch W. Laccase immobilization on copolymer of butyl acrylate and ethylene glycol dimethacrylate. Biochem Eng J. 2007;35:325–327. [ Google Scholar ]
- 123. Palm AK, Novotny MV. Analytical characterization of a facile porous polymer monolithic trypsin microreactor enabling peptide mass mapping. Rapid Commun Mass Spectrom. 2004;18:1374–1379. doi: 10.1002/rcm.1500. [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. Freije JR, Mulder PPMFA, Werkman W, Rieux L, Niederlander HAG, Verpoorte E, Bischoff R. Chemically modified, immobilized trypsin reactor with improved digestion efficiency. J Proteome Res. 2005;4:1805–1808. doi: 10.1021/pr050142y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 125. Goradia D, Cooney J, Hodnett BK, Magner E. Characteristics of a mesoporous silicate immobilized trypsin bioreactor in organic media. Biotechnol Prog. 2006;22:1125–1131. doi: 10.1021/bp050334y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 126. Ota S, Miyazaki S, Matsuoka H, Morisato K, Shintani Y, Nakanishi K. High-throughput protein digestion by trypsin-immobilized monolithic silica with pipette-tip formula. J Biochem Biophys Methods. 2007;70:57–62. doi: 10.1016/j.jbbm.2006.10.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 127. Bencina K, Podgornik A, Strancar A, Bencina M. Enzyme immobilization on epoxy- and 1,1′-carbonyldiimidazole-activated methacrylate-based monoliths. J Sep Sci. 2004;27:811–818. doi: 10.1002/jssc.200401800. [ DOI ] [ PubMed ] [ Google Scholar ]
- 128. Monzo A, Sperling E, Guttman A. Proteolytic enzyme immobilization techniques for MS-based protein analysis. TrAC Trends Anal Chem. 2009;28(7):854–864. [ Google Scholar ]
- 129. Stigter ECA, de Jong GJ, van Bennekom WP. Development of an open-tubular trypsin reactor for on-line digestion of proteins. Bioanal Chem. 2007;389:1967–1972. doi: 10.1007/s00216-007-1584-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 130. Couto RS, Herrera TJL. Industrial and biotechnological applications of laccases: a review. Biotechnol Adv. 2006;24:500–513. doi: 10.1016/j.biotechadv.2006.04.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 131. Kunamneni A, Plou FJ, Ballesteros A, Alcalde M. Laccases and their applications: a patent review. Recent Pat Biotechnol. 2008;2:10–24. doi: 10.2174/187220808783330965. [ DOI ] [ PubMed ] [ Google Scholar ]
- 132. Riva S. Laccases: blue enzymes for green chemistry. Trends Biotechnol. 2006;24:219–226. doi: 10.1016/j.tibtech.2006.03.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 133. Xu F. Applications of oxidoreductases: recent progress. Ind Biotechnol. 2005;1:38–50. [ Google Scholar ]
- 134. Claus H, Faber G, König H. Redox-mediated decolorization of synthetic dyes by fungal laccases. Appl Microbiol Biotechnol. 2002;59:672–678. doi: 10.1007/s00253-002-1047-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 135. Rochefort D, Leech D, Bourbonnais R. Electron transfer mediator systems for bleaching of paper pulp. Green Chem. 2004;6:14–24. [ Google Scholar ]
- View on publisher site
- PDF (1.0 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Immobilization of Enzyme
Definition : Immobilization can define as the enticement of an enzyme to a solid support. Enzyme immobilization is a process, which encloses the enzyme molecules to an absolute phase from a bulk phase. The bulk phase consists of substrates, effectors and inhibitors. Enzyme molecule impounds in or on some suitable matrix that is having a definite porosity.
The carrier or matrix allows the exchange of medium (contains substrate and product), to which an enzyme molecule can be confined. Immobilization restricts the movement of enzymes. It is a prevalent method, which is now used in many fields like industrial, medical, bioresearch, food science etc.
Therefore, the biocatalyst that gets confined to the inert material will be termed as an immobilized enzyme. The methods which facilitate the enzyme entrapment into or on the support matrix is called immobilization of an enzyme. Amino acylase was the first immobilized enzyme.
Here, we will look into some important topics related to the context like the discoveries behind the introduction of the immobilization technique, the components, physical and some chemical methods of immobilization. Besides, we will also discuss some of the advantages, disadvantages and applications of the immobilization technique.
Content: Immobilization of Enzyme
Encapsulation, covalent binding, cross linking, advantages of immobilization, disadvantages of immobilization, applications of immobilized enzymes.
History of enzyme immobilization includes three stages in the development of an immobilized enzyme.
- First stage : During 1815, immobilized enzymes were used empirically in industrial processes, such as the production of acetic acid and wastewater treatment.
- Second stage : During the 1960s, a single enzyme immobilization system was introduced. Then this system used in the production of L-amino acids, isomerization of glucose etc.
- Third stage : During 1970-1995, multiple enzyme immobilization methods, including co-factor regeneration and cell immobilization, were introduced.
Components of Enzyme Immobilization
It can define as a biomolecule, which accelerates many biochemical reactions. It acts as a biocatalyst, which mediates the conversion of a substrate into a product, but never used up by the substrate.
Support Matrix
It is a material, which facilitates the imprisonment of an enzyme. A support matrix must have the following properties that we have discussed below for the efficient immobilization.
Ideal Properties of Support Matrix
- Mechanical strength.
- Biocompatible
- Regenerability
- Ease of derivatization (transformation of the substrate into a product)
- Expansion of enzyme specificity
- Reduction in product inhibition
- Reduction of microbial contamination
- Cost-effective
Classification of a Matrix
Organic matrix: It subdivides into natural and synthetic polymers.
- Natural polymers : It shows favourable compatibility with proteins. Polysaccharides (Cellulose, dextran, agar, agarose, chitin, alginate etc.), proteins (Collagen, albumin) and carbon are the natural polymers.
- Synthetic polymers : It shows high chemical and mechanical stability. Polystyrene, polyacrylate, polyacrylamide, polyamides etc. are examples of synthetic polymers.
Inorganic matrix: It subdivides into Natural and Processed minerals.
- Natural minerals : E.g.Bentonite, celite, centolite, silica, charcoal etc.
- Processed materials : E.g.Porous glass, metals and metal oxides.
Physical Characteristics of the Matrices
- Mean particle size
- Hydrophilic character
- Mechanical strength
Mean particle size : It is the parameter, which decides the porosity of a carrier. Porous matrix prefers over the non-porous matrix because it increases the surface area, by which the loading capacity of an enzyme also increases. This property of the matrix determines the total surface area and also affect the binding ability of a catalyst. A porous material should have optimized pore distribution to optimize the flow of particles.
Hydrophilic character : It determines the level of activity by an immobilized enzyme.
Mechanical strength : It can define as the binding of an enzyme that is inversely related to the ease with which it gets entrapped.
Mode of attachment
The different modes of attachment between the enzyme and support matrix are explained below in the methods of enzyme immobilization. It generally involves physical and chemical methods for the immobilization of an enzyme.
Methods of Enzyme Immobilization
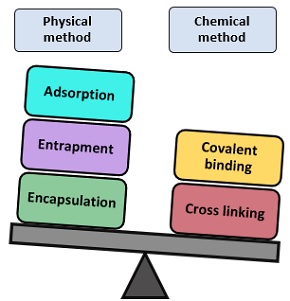
It is the oldest and simplest method. In this type, enzyme adheres to the surface of the water-insoluble carrier matrix. The binding is nonspecific like electrostatic or hydrophobic affinity binding to a particular ligand. The binding between enzymes and the carrier matrix is usually firm, but it gets weakened by many factors like:
- Addition of substrate
- pH or ionic strength
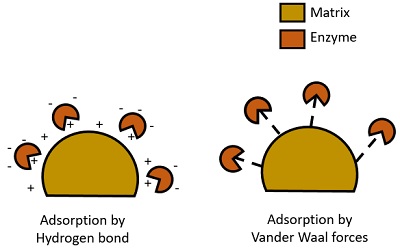
- The matrix used : The matrix’s particle size must be small (500Å-1mm D).
- Mineral support (E.g. Aluminium oxide, clay)
- Organic support (E.g. Starch)
- Modified sepharose and ion exchange resins
Methods of Immobilization by Adsorption :
- Static method : It is an efficient but time-consuming method. It involves immobilization of enzyme and carrier molecule without agitation.
- Dynamic process : It involves the mixing of an enzyme with the carrier, under constant agitation.
- Reactor loading : It involves transferring of both enzyme and carrier in the reactor with the agitation of the whole content. It is widely used for the commercial production of the immobilized enzyme.
- Electro-deposition : Here, a carrier is kept proximal to the electrode in an enzyme bath, after which an electric current is passed through it. It results in the movement of an enzyme towards the carrier. At last, the enzyme gets deposited on the surface.
Advantages of Adsorption :
- It has no pore diffusion limitation.
- It is a simple and economical method to conduct.
- No reagents are required in this method.
- There is a limited loss of enzyme activity.
- It causes less disruption to an enzyme.
- It requires minimum activation steps.
- The adsorped enzyme can be recycled, regenerated and reused.
- It has a high loading efficiency of an enzyme.
Disadvantages of Adsorption :
- It provides low surface area for the enzyme binding.
- Desorption of an enzyme from the carrier usually occurs.
- Also, the yield is low.
An enzyme traps inside a porous polymer or gel matrix during this method. It is also called lattice entrapment . The bonding between an enzyme and matrix can be covalent or non-covalent.
- The matrix used : It is water-soluble, and nature varies with different enzymes.
- Examples : It makes the use of the following carrier materials like polyacrylamide gels, cellulose triacetate, agar, gelatine, alginate etc.
Methods of enzyme entrapment : It involves the inclusion of an enzyme into the following matrices:
- Gels : Involves entrapment of an enzyme inside the gel matrix.
- Fibres : Entrapment of an enzyme inside the fibre matrix.
- Microcapsules : Involves entrapment inside a microcapsule.
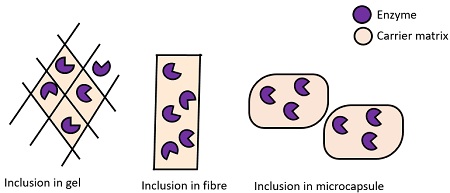
Advantages of Entrapment Method :
- Its enzyme loading capacity is high.
- It’s a rapid method.
- Here, the enzyme distortion is low.
- It is easy to practice.
Disadvantages of Entrapment Method :
- The diffusion of substrate and product create difficulties.
- It causes leakage of low molecular weight enzymes.
- There might some chances of microbial contamination.
- It also causes enzyme inactivation and sometimes loss of enzyme activity.
- It has limited industrial use.
It is the membrane confinement method. An enzyme confines within the semipermeable membrane of a capsule in an aqueous solution. This process allows the exchange of medium (substrate & product), but not an enzyme. The effectiveness of encapsulation relies upon the enzyme stability.
- The matrix used : The capsule is made of a semi-permeable membrane, and it can be polymeric, lipoid, non-ionic etc. in nature.
- Examples : It includes nitrocellulose, nylon semi-permeable matrix etc.
Methods of encapsulation : It can be achieved by the following ways:
- Encapsulation in a reaction vessel : It involves the partitioning of a chamber by a semipermeable membrane. One chamber contains enzymes, whereas the other contains substrate and product.
- Encapsulation by hollow fibre membrane : It involves entrapment of an enzyme inside a semipermeable matrix (cellulose, triacetate etc.). Here, an enzyme traps inside the space of the matrix.
- Microencapsulation : By chemical polymerization, the enzyme molecules enclose inside a microcapsule by the use of 1-6-diaminohexane.
- Encapsulation by liposomes : Here, an enzyme binds to the concentric lipoidal membrane of the liposome by the use of phospholipid.
Advantages of Encapsulation :
- There is no enzyme leakage.
- It does not affect enzyme activity.
- It’s a simple method to conduct.
- It possesses a high loading efficiency of an enzyme.
Disadvantages of Encapsulation :
- It makes the use of a carrier that has a pore size limitation.
- It is not so cost-effective.
It is a widely used method. An enzyme molecule binds to the carrier by a covalent bond during this process. Here, the binding strength is powerful or a complex form through this bonding is stable. Also, there is no enzyme loss during the process. Covalent binding occurs between the active part, i.e. the functional group of an enzyme and the carrier molecule.
The functional group that are participating in the binding process are -NH 2 , -NH 3, -COO, -OH, -SH, -O, -S etc. The order of reactivity of these functional groups to the carrier depends upon their charged status: -S – > -SH > -O – > -NH 2 > -COO – > -OH >> -NH 3 +
Examples of a polymeric carrier used in covalent binding are as follows:
- Carboxylic acid and related groups of polyglutamic acid
- Amide group of a polypeptide
- Amino and related groups of polysaccharides
- Some most commonly used polymers are the polysaccharide (celluloses, agarose, sepharose, etc.), polyvinyl alcohol, silica and porous glasses.
Methods used for Covalent Binding : It involves the following methods that are given below.
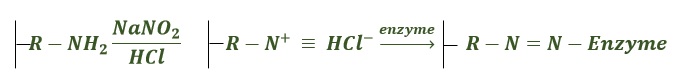
- By polyfunctional reagents : This process involves bonding between the amino group of the matrix and amino group of an enzyme. Example: Glutaraldehyde (Bi-functional reagent).
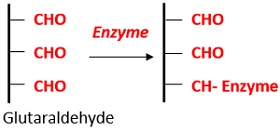
Advantages of Covalent Binding :
- It provides a high binding strength between an enzyme and a carrier.
- It’s a simple and widely used method.
- The process is not affected by the pH or ionic strength.
Disadvantages of Covalent Binding :
- Denaturation of the enzyme occurs during the immobilization.
- Only a small amount of enzyme can be immobilized.
It is also called “ Copolymerization “. Here, the immobilized enzymes covalently link to the various groups of an enzyme via polyfunctional reagents. It does not require a support matrix. Cross-linking leads to the formation of 3D crosslinked aggregates. The most commonly used polyfunctional agents are glutaraldehyde and diazonium salts etc.
Advantages of Cross Linking Method :
- There is little or no enzyme leakage.
- It yields a highly stabilized enzyme.
- It’s a simple and cheap method to carry out.
- It has wide applicability in the commercial production of the enzyme.
Disadvantages of Cross Linking Method :
- It causes enzyme inactivation.
- The polyfunctional reagents used in this process generally cause enzyme denaturation.
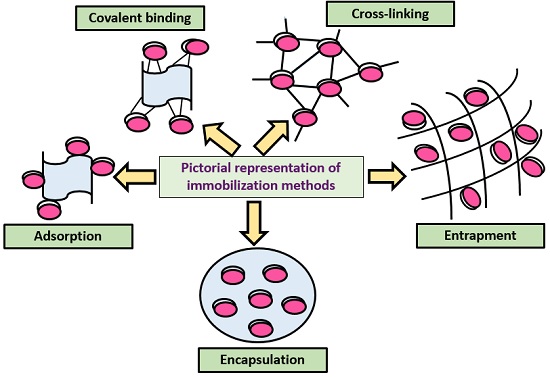
- The enzyme can be reused.
- The immobilization method minimizes the labour input.
- It increases the enzyme and substrate ratio.
- It requires a minimum activation time.
- Its industrial use is limited.
- The enzymes may lose their catalytic property and stability.
- The enzymes get inactivated by the heat generation.
- It is expensive to conduct this method.
- Industrial use : E.g. Production of antibiotics, amino acids etc.
- Biomedical use : E.g. For the treatment, diagnosis and drug delivery.
- In the food industry : E.g. Production of jams, jellies, syrups etc.
- Sewage treatment : E.g. In the treatment of sewage and industrial effluent for wastewater management.
- In the detergent industry : E.g. Immobilization of lipases to digest lipid present in stains or dirt.
- Textile industry : E.g. Scouring, bio-polishing etc.
Thus immobilization process is widely used in various fields, in which an enzyme can be immobilized to the particular phase after which it can be reused and stabilized to carry out many reactions.
Related Topics:
- Tikka Disease of Groundnut
- Acetic Acid Production
- Activated Sludge Treatment
- Maintenance and Preservation of Pure Culture
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Start typing and press enter to search

IMAGES
VIDEO