

The Biology Corner
Biology Teaching Resources

Observing Diffusion Using Iodine and Plastic Bags
Most chapters follow the cell structure topic with one on the cell membrane and diffusion and osmosis. These concepts can be very difficult for students to understand.
In order to give them a view of how diffusion works with a semipermeable membrane, I like to do a lab that uses a plastic bag to model the cell (membrane).
It is a simple lab where students do very little except watch the process and record data and information. To set it up, you will need plastic bags, iodine, water, and corn starch. All except iodine are readily available at the supermarket.
Student handout includes instructions, pre-lab questions, and an analysis section.
Setting Up the Lab
First, add a spoonful of cornstarch and about 100 ml of water in a cheap plastic bag. (You don’t need to be exact!) Tie the top of the bag like a balloon. I like to do at least one of these while the students watch. Then I give them prepared bags because it takes too long (and is messy) for students to do this step.

Tell the students that the bag represents a cell, with the cytoplasm being the cornstarch mixture and the plastic is the cell membrane. Explain that solid objects are not really solid at a molecular level and that the bag is more like a tiny little screen door. If molecules are small enough, they can pass right through the bag.
With the baggie in place, you will need to prepare enough beakers for your entire class. Fill them about half full and add several drops of iodine , You want the water to be very orange. The more concentrated the mixture, the faster the reaction.

Observations and Analysis
Explain that iodine is an “INDICATOR” in that it will change color whenever it encounters starch. You can demonstrate this with a beaker of starch solution and a drop of iodine. Usually students are fascinated by the quick and dramatic color change!
Finally, students will carefully place the baggie into the iodine mixture. Their worksheet will ask them to make some predictions about what will happen and to define diffusion and osmosis. The process should take about 15 minutes and students should notice a change in the color of the corn starch in the bag.

After 15 Minutes….

Students will then be asked on the worksheet to explain what happened. A common misconception is that the iodine “ate” through the bag. Remind students that the bag is like a screen door and iodine is a very small molecule.
Despite the fact that this lab is not very interactive, students do seem to understand the cell model and semi-permeable membranes after completing it. Many will ask to see what will happen if you put the starch in the beaker and the iodine in the bag. You can set this up for them and they can view the results the next day.
Shannan Muskopf

Search My Blog
Very simple diffusion and osmosis experiment.

5 comments:
Good blog. Very easy to understand the point of each piece of text.

How do you make your starch solution and glucose solution?

Since no quantitative data is being collected, there is no need to make solutions of a specific concentration. I put some corn starch in a beaker of water and boil it until it clears up a bit. I put several teaspoons of Karo syrup in a beaker of water and stir until throughly mixed.
where can i find these dialysis tubing??
We order them with our lab supplies. You can also use a plastic sandwich bag.

Learning Objectives
After completing the lab, the student will be able to:
- Explain or define the term diffusion.
- Explain how different media affect the rate of diffusion.
Activity 1: Pre-Assessment
- What happens when an air freshener is sprayed in a corner? What is the name of the process that causes the molecules to move?
- Do you think that the rate of the air freshener molecules moving would change if the room temperature was warmer or colder? Why or why not?
- Discuss the answers to questions 1 and 2 with the class.
Activity 1: Diffusion
The movement of molecules from a higher concentrated area to a wider and less concentrated area is referred to as diffusion . For example, you can smell the aroma of food flowing through the atmosphere as you walk towards a cafeteria. Molecules collide with each other and are in constant motion because of their kinetic energy. This activity propels molecules to move where there is a less concentrated area. Therefore, the net movement of molecules is always from a tightly concentrated area to a less tightly packed area. Osmosis is the process of water diffusion through a selectively permeable membrane. In body systems, various constituents such as gases, liquids, and solids are dissolved in water when they flow through the cell membrane from a highly concentrated place to a less concentrated area in bodily systems. In a solution, the dissolved substance is called the solute and the substance in which the solute is dissolved is called the solvent.
Diffusion is the movement of molecules from an area where the molecule is highly concentrated to an area of low concentration, as illustrated in Figure 6.1. The rate of diffusion is dependent upon the temperature of a system, molecular size, and the medium through which diffusion is occurring (i.e., semi-solid, liquid, air). In this activity, we will be observing the diffusion of a dye through a beaker of water and through agar (a gelatinous substance), diffusion as a function of temperature, and diffusion as a function of molecular weight.
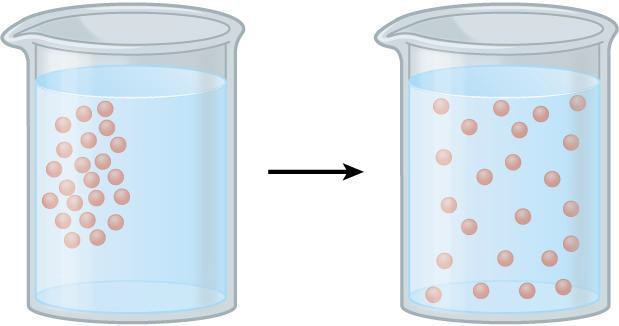
Safety Precautions
- Inform your teacher immediately of any broken glassware, as it could cause injuries.
- Clean up any spilled water or other fluids to prevent other people from slipping.
- Be careful with the dye as it can stain your clothes, and it should not be ingested.
- Wash your hands with soap and water after completion of the activity.
For this activity, you will need the following:
- Three 250 mL beakers
- Food coloring
- Agar plates
- Potassium permanganate
- Methylene blue
- Thermometer
- Refrigerator
- Clock or timer
For this activity, you will work in groups of four .
Structured Inquiry
Step 1: Measure 200 mL of room temperature water in a beaker. Put three drops of food coloring into the water. Time how long it takes for the dye to completely diffuse throughout the water. Record the time and describe in your notebook what you observe. Create a data table for your observations.
Step 2: Hypothesize/Predict: Predict what would happen to the rate of diffusion if you had beakers with both very hot and very cold water in them. Add your predictions to the data table you created in step 1.
Step 3: Student-led Planning: Determine how diffusion of the food color would be affected when the water is either very hot or very cold. Use a thermometer and record the temperature for each. Use a timer to measure how long it takes for complete diffusion to occur in all scenarios.
Step 4: Critical Analysis: Create a graph that shows how the diffusion rate is affected because of temperature change. Are the predictions you made in step 2 supported by your data? Why or why not? What methods could you use to improve your results? Discuss with your group and then write your answers in your notebook.
Guided Inquiry
Step 1: Gather four agar plates and the three dyes, provided by your instructor, that differ in molecular size: Congo red (mol. wt. 696.66 g/mol), methylene blue (319.85 g/mol), and potassium permanganate (mol. wt. 158.03).
Step 2: Hypothesize/Predict: How would the rate of diffusion of a molecule through a gel compare to its rate of diffusion through water? How would the rate of diffusion differ between molecules of different molecular sizes? Write your ideas in your notebook.
Step 3: Student-led planning: Use 1 plate for determining how molecular size affects diffusion using the 3 dyes. Determine how best to measure movement of the dye in an agar plate. Be sure to keep the dyes far enough apart so that they do not touch once they start diffusing. Get your instructor’s approval before proceeding with the experiment. Measure the distance that the dye spreads in 20-minute intervals for 1 hour.
Step 4: Examine the effect of temperature on the rate of diffusion for 1 dye of your choosing. With your group, determine 3 temperatures that would be appropriate. Measure the diameter of the dye spread for each. Write the results in your notebook.
Step 5: Critical Analysis: Rank all 3 dyes in terms of diffusion rate. What was the relationship between diffusion rate and molecular size? What is the relationship between temperature and diffusion rate? Discuss your answers with your group and write them in your notebook.
Assessments
- In a system, there is a concentration of molecules. However, on the outside, there is little to no concentration of this particular molecule. In which direction would the molecules be moving more so than the other direction?
- Diffusion is affected by what factors?
- Dye tends to move faster in warmer temperatures. Why is this?
Lab Manual for Biology Part I Copyright © 2022 by LOUIS: The Louisiana Library Network is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
6 Lab 5. Diffusion and Osmosis
Lab 5—diffusion & osmosis.
- Practice applying hypothesis testing, and further your understanding of the basic principles underlying the scientific method and experimental design.
- Identify independent and dependent variables in an experiment.
- Describe diffusion and osmosis.
- Practice graphing data obtained in the lab and designing useful data tables.
- Understand and be able to define the following terms: diffusion, osmosis, concentration gradient, tonicity, hypotonic, hypertonic, isotonic, turgor, selectively permeable, semipermeable, osmotic pressure, turgor pressure, plasmolysis, hypothesis, prediction, theory, control, independent variable, dependent variable, controlled variable, quantitative variable, qualitative variable.
- Draw a “flow chart” (in your lab notebook) to diagram what you will do (and in what order) in this Lab.
Exercise A – Plasmolysis in green leaf cells
- Predict what you think will happen and why, when the fresh water around the leaf is replaced by salt water. Predict what will happen with respect to osmosis and also with respect to what will happen to the cell itself.
Exercise B – Osmosis and dialysis tubing
- Predict what will happen inside the tubing comparing the three concentration treatments.
Exercise C – ‘Osmosis Egg as Model Cell’
- What hypothesis is being tested by this “experiment”/demonstration?
- What is the dependent variable?
- What is the independent variable?
- Are they quantitative or qualitative?
EXERCISE A – INTRODUCTION
In this lab you will explore the processes of diffusion and osmosis. Diffusion can occur across a semipermeable membrane, however diffusion also occurs where no barrier (or membrane) is present. A number of factors can affect the rate of diffusion, including temperature, molecular weight, concentration gradient, electrical charge, and distance. Water can also move by the same mechanism. This movement of water is called osmosis .
- Please work in pairs for Exercises A and B and work in groups of 4 for Exercise C.
- BEFORE YOU BEGIN make a group plan about what you will do and who will be doing what. Your Flow Chart that you made for the Prelab Assignment will be useful for this. Note that one of the exercises requires using a microscope and each student should make their own microscopic observations and drawing.
- All data/observations must be written in your lab notebook.
Exercise A: Plasmolysis – Osmosis in a Living System (plant leaf)
Plant cells rely on pressure exerted outwardly by the fluid in the their vacuole to help them maintain their shape, much as a tire must be pumped up with air to provide the outward pressure that maintains the tire’s shape. The pressure exerted by the vacuole’s fluid is called turgor pressure . That pressure is maintained by the cell by adjusting the concentration of solutes in the vacuole’s fluid. In this exercise you will not be testing a hypothesis. Instead, you will observe what happens when you replace the fresh water surrounding a specimen of plant cells with a salt solution.
1) Obtain a small section of a leaf from an aquatic plant and put it on a clean slide. Place a drop of water on the specimen on the slide, cover it with a cover slip, and examine the leaf first at scanning (40X), the low power (100X) and then at high power (400X). Locate a region of healthy cells where there are only one or two layers of cells. Be sure that you can clearly see the individual cells. Sketch several adjacent cells at 400x in your lab notebook. Remember to include total magnification and a title that identifies the organism. Label the structures that you can see such as cell walls, nuclei, vacuoles, and chloroplasts. DO NOT move the slide while doing the next step; you will want to observe the same cells.
2) While touching one edge of the cover slip with a piece of Kimwipe to draw off the water, add a drop of 15% salt solution to the slide next to the opposite edge of the cover slip. Be sure that the salt solution moves under the cover slip. Observe how the cell responds. After a few minutes, sketch the same cells you sketched in before (in step a). Label the cell structures again, including the cell or plasma membrane. Be sure to label both sketches “before adding saltwater” and “after adding saltwater”.
Include in your lab notebook answers to the following questions:
- Describe your observations (i.e. what happened?) when the water in which the cells were mounted was replaced by the salt solution. Refer to the cell structures that you labeled.
- Assuming that the cells have not been killed, what should happen if the salt solution were to be replaced by water? Describe what you would likely observe (i.e., make a prediction), and explain.
- Can osmosis likely cause plant cells to burst? Explain, comparing plant and animal cells.
For photomicrographs of images similar to what you observed in lab, you can do a “Google” image search for ‘Elodea plasmolysis’. These images will not be the same as your observations and drawings that you must do from your slides.

EXERCISE B – INTRODUCTION
Exercise b: demonstrating osmosis using semi-permeable tubing.
The water movement through a membrane is called Osmosis .
All living cells are surrounded by a selectively permeable membrane, which contains transmembrane proteins with hydrophilic interiors (channels) to allow smaller polar molecules to pass such as water, ions, sugars and amino acids. Especially favored is the movement of water molecules through many aquaporins or water channels.
In this exercise, you will use synthetic or human-made membranes, which were first developed in Seattle, and used in artificial kidneys or dialysis machines. We will use them to demonstrate the osmotic movement of water molecules into concentrated sugar solutions.
Materials: (per group of 2)
- One large tray
- One large beaker (500mL)
- Three 16cm dialysis tubes
- Ring stand with clamps x3
- Three 10mL Falcon Tubes
- Three stretches of thread or cord
- Green tubing clamp (flat)
In this portion of the lab, please work in groups of 2 at your lab bench.
- Cut three 16-cm-long strips from the dialysis tube. It is dry and flat and needs to be soaked in warm water.
- Get three 10 ml-sized Falcon Tubes and remove the lower tip with a razor blade or scissors while leaving a cone-shaped opening.
- You now push one open end of the wet dialysis tubing over the cone-shaped end of the Falcon Tube. It will resist at first but finally fit snugly over the tube since both have similar diameters. Wrap some thread or cord around the tube fixing it firmly to the Falcon tube.
- At the other end of the dialysis tube you fold it twice and clamp it closed with a green tubing clamp. This should be done so that you have an exposed length of dialysis tube of about 10 cm. Attach the Falcon tube to a ring stand and a clamping fixture.
Fill it slowly with tap water to test for stability and potential leaks.
- Repeat this procedure to produce two more identical sets.
- You are now ready to fill the three tubes with different solutions. One tube will contain a Pancake syrup (fully or 1.0 x concentrated), another tube with diluted Pancake syrup (0.5 x concentrated), and the third tube with water (as a control). Fill all three tubes to a similar height and mark the upper meniscus with black marker line.
- You are set to start the experiment by lowering the filled dialysis tubes completely into a large (500 ml) beaker with water. Record time zero in the table below.
DATA ANALYSIS & INTERPRETATION
- Graph the results with 3 curves (placet the dependent variable on the y axis). Make sure to include a legend to tell the differences between treatments.
- Why does the water flow into the dialysis tubes rather than the sugar flowing out and into the water of the beaker?
- If the Falcon tubes would be closed, the liquid level could not rise. What would happen in this case? Then, compare what might happen if this was an animal cell.
EXERCISE C – OSMOSIS & TONICITY
Materials: (per group of 4)
- 1 large tray • 3 – 400 ml beakers
- 3 weigh boats • 1 electronic balance
- 3 decalcified eggs • paper towels
In this portion of the lab, please work in the same groups of four at your lab bench.
There will be three solutions available in lab. Your objective is to determine the tonicity of these solutions relative to the egg “model cells”. These solutions may be isotonic, hypotonic or hypertonic relative to the egg. Models or simulations are used extensively in science to study phenomenon that may otherwise be difficult to study. We will model cells by using eggs. The egg shells have been removed (decalcified) by soaking them in vinegar, and the remaining egg membrane is permeable to water but not sugar.
Work over the large tray when you are handling the eggs!
Your group should choose three solutions for your three beakers. You will dry and weigh the three eggs before placing them in the beakers. As the egg sits in the solution, it will either gain weight, lose weight, or remain the same weight. By weighing the eggs, you will be able to determine the tonicity of each unknown solution relative to each egg. Each egg should be in solution at total of 30 minutes. Your group will need to determine how often to weigh your eggs. You will need at least 5 measurements for each egg in each solution, more may be better. For your measurements, you will gently remove from its solution (work over the tray) and weigh every X minutes.
Include the following in your lab notebook:
- What hypothesis is being tested by this experiment? Remember that this should mention both the independent and dependent variables and explain the phenomenon.
- Remember to use the “If … then …” format. Remember that the “then…” part is stated with respect to the dependent variable (what you predict will happen to the dependent variable). Explain why you made your prediction based on your knowledge from lecture, readings, etc.
- What is the dependent variable? Is it a quantitative or qualitative variable?
- What is the independent variable? Is it a quantitative or qualitative variable?
- Record all of your experimental data in the table(s) that you created for your prelab.
- Using the wax pencil, label beakers, to identify the solution(s), for example solution “A”, solution “B” or solution “C”. Fill each beaker half full (approximately 200 ml) with the appropriate solutions (A, B or C). Also label the threeweigh boats (A, B, or C) with a wax pencil.
- Carefully dry off the eggs. Handle the eggs one at a time OVER the tray throughout the experiment, in case the egg breaks!
- Carefully weigh each egg (A, B or C) in its weigh boat.
- Record the initial weights of the egg in solution A, B and/or C in data tables in your lab notebook (remember to include units).
- Carefully place each egg in its corresponding beaker. Note the position of each egg in its respective solution. Record the time at which you placed each egg in its solution.
- After each egg has been in its solution for the required amount of time, carefully remove the egg from the beaker. Working over the tray (in case the egg breaks), dry off the egg with paper towels, and weigh the egg in its weigh boat.
- Collect and record your data. Record the times and weights in the data table in your lab notebook.
- Note the final weight of each egg compared to the initial weight. What is the difference for each egg.
DATA ANALYSIS
- Complete your data tables (in your lab notebook).
- Graph these data by hand in your lab notebook. Each student must produce their own graph. Include the weight of each egg (in grams), time or time elapsed (in hours:minutes or minutes), a key, and an informative caption.
INTERPRETATION
- What are the tonicities of your three solutions relative to the solutions inside the cells? Explain your conclusion.
- What can you conclude about the rates of osmosis, based on your data and your graph?
- Is the rate constant (over 30 minutes or more) for each egg?
- What do you think would happen if you allowed the egg to sit in each solution for hours?
ERROR ANALYSIS
- What mistakes occurred? If none occurred, what mistakes could have occurred?
- What other sources of error can you think of, besides any mistakes?
LWTech General Biology (BIOL&160) Lab Protocols Copyright © by Lake Washington Institute of Technology is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book
Optional Lab Activities
Osmosis and diffusion, lab objectives.
At the conclusion of the lab, the student should be able to:
- define the following terms: diffusion, osmosis, equilibrium, tonicity, turgor pressure, plasmolysis
- describe what drives simple diffusion (why do the molecules move?)
- list the factors that may affect the speed of simple diffusion
- list which molecules, in general, can freely diffuse across the plasma membrane of a cell
- describe what drives osmosis (why do water molecules move?)
- explain why water moves out of a cell when the cell is placed in a hypertonic solution
- explain why water moves into a cell when the cell is placed in a hypotonic solution
- describe what physically happens to a cell if water leaves the cell
- describe what physically happens to a cell if water enters the cell
http://www.slideshare.net/CandelaContent/membrane-lab
Introduction
Understanding the concepts of diffusion and osmosis is critical for conceptualizing how substances move across cell membranes. Diffusion can occur across a semipermeable membrane; however diffusion also occurs where no barrier (or membrane) is present. A number of factors can affect the rate of diffusion, including temperature, molecular weight, concentration gradient, electrical charge, and distance. Water can also move by the same mechanism. This diffusion of water is called osmosis .
In this lab you will explore the processes of diffusion and osmosis. We will examine the effects of movement across membranes in dialysis tubing, by definition, a semi-permeable membrane made of cellulose. We will also examine these principles in living plant cells.
Part 1. Diffusion Across a Semi-Permeable Membrane: Dialysis
- Cut a piece of dialysis tubing, approximately 10 cm.
- Soak the dialysis tubing for about 5 minutes prior to using.
- Tie off one end of the tubing with dental floss.
- Use a pipette and fill the bag with a 1% starch solution leaving enough room to tie the other end of the tubing.
- Tie the other end of the tubing closed with dental floss.
- Fill a 250 mL beaker with distilled water.
- Add Lugol’s iodine to the distilled water in the beaker until the water is a uniform pale yellow color.
- Place the dialysis tubing bag in the beaker.
- The movement of starch
- The movement of iodine
- The color of the solution in the bag after 30 minutes
- The color of the solution in the beaker after 30 minutes
- Add the dialysis bag to the beaker and allow the experiment to run for 30 minutes. Record the colors of both the dialysis bag and the beaker.
Lab Questions
- Is there evidence of the diffusion of starch molecules? If so, in which direction did starch molecules diffuse?
- Is there evidence of the diffusion of iodine molecules? If so, in which direction did iodine molecules diffuse.
- What can you say about the permeability of the dialysis membrane? (What particles could move through and what particles could not?)
- What is the difference between a semi-permeable and a selectively permeable membrane
Part 2. Plasmolysis—Observing Osmosis in a Living System, Elodea
If a plant cell is immersed in a solution that has a higher solute concentration than that of the cell, water will leave/enter (circle one) the cell. The loss of water from the cell will cause the cell to lose the pressure exerted by the fluid in the plant cell’s vacuole, which is called turgor pressure. Macroscopically, you can see the effects of loss of turgor in wilted houseplants or limp lettuce. Microscopically, increased loss of water and loss of turgor become visible as a withdrawal of the protoplast from the cell wall (plasmolysis) and as a decrease in the size of the vacuole (Figure 1).
- Obtain a leaf from the tip of an Elodea Place it in a drop of water on a slide, cover it with a coverslip, and examine the material first at scanning, then low power objective and then at high power objective.
- Locate a region of health. Note the location of the chloroplasts. Sketch a few cells. For the next step, DO NOT move the slide .
- While touching one corner of the coverslip with a piece of Kimwipe to draw off the water, add a drop of 40% salt solution to the opposite corner of the coverslip. Do this simultaneously. Be sure that the salt solution moves under the coverslip. Wait about 5 minutes, then examine as before. Sketch these cells next to your sketch of cells in step two, note the location of the chloroplasts. Label it 40% salt solution .
- What happened to the cells in the salt solution?
- Assuming that the cells have not been killed, what should happen if the salt solution were to be replaced by water?
- Are plant cells normally hypertonic, hypotonic, or isotonic to their environment? Why?
- Can plant cells burst? Explain.
Overall Conclusions
- Review your hypothesis for each experiment. Was your original hypothesis supported or rejected for each experiment. Explain why or why not. This should be based on the best information collected from the experiment. Explain how you arrived at this conclusion.
- If it was incorrect, give the correct answer, again based on the best information collected from the experiment.
Sources of Error
- Identify and explain two things that people may have done incorrectly that would have caused them to get different answers from the rest of the class. Be specific .
Candela Citations
- Biology 101 Labs. Authored by : Lynette Hauser. Provided by : Tidewater Community College. Located at : http://www.tcc.edu/ . License : CC BY: Attribution
- BIOL 211 - Majors Cellular [or Animal or Plant]. Authored by : Carey Schroyer and Diane Forson. Provided by : Open Course Library. Located at : http://opencourselibrary.org/biol-211-majors-cellular-or-animal-or-plant/ . License : CC BY: Attribution

IMAGES
COMMENTS
The following points highlight the top five experiments on diffusion. The experiments are: 1. Diffusion of Solid in Liquid 2. Diffusion of Liquid in Liquid 3. Diffusion of Gas in Gas 4. Comparative Rates of Diffusion of Different Solutes 5. Comparative rates of diffusion through different media.
Practical 1: Investigating the rate of diffusion using visking tubing. Visking tubing (sometimes referred to as dialysis tubing) is a non-living partially permeable membrane made from cellulose; Pores in this membrane are small enough to prevent the passage of large molecules (such as starch and sucrose) but allow smaller molecules (such as glucose) to pass through by diffusion
In order to give them a view of how diffusion works with a semipermeable membrane, I like to do a lab that uses a plastic bag to model the cell (membrane). It is a simple lab where students do very little except watch the process and record data and information. To set it up, you will need plastic bags, iodine, water, and corn starch.
The concept of cellular transport (diffusion, osmosis, hypotonic, hypertonic, active transport, passive transport) is fundamental to a biology class.There are so many great ideas for labs that teach and explore these concepts. Just this week, our biology students completed an activity that is so very simple, but it really illustrates the concept of semipermeable membranes.
This is diffusion. The rate of diffusion is influenced by both temperature (how fast the particles move) and size (how big they are). Part 1: Brownian Motion . In this part of the lab, you will use a microscope to observe Brownian motion in carmine red powder, which is a dye obtained from the pulverized guts of female cochineal beetles. Materials
In this activity, we will be observing the diffusion of a dye through a beaker of water at different temperatures. Figure 7.1: In diffusion, molecules move from areas of high concentration to areas of low concentration. Safety Precautions. Inform your teacher immediately of any broken glassware, as it could cause injuries.
Lesson&Plan:&Diffusion&! Background! Particles!in!cells!show!rapid!back!and!forth!movement,!or!Brownian!motion,which!isalsoknownas!diffusion.!The!back! and!forth ...
Diffusion is the movement of molecules from an area where the molecule is highly concentrated to an area of low concentration, as illustrated in Figure 6.1. The rate of diffusion is dependent upon the temperature of a system, molecular size, and the medium through which diffusion is occurring (i.e., semi-solid, liquid, air).
In this lab you will explore the processes of diffusion and osmosis. Diffusion can occur across a semipermeable membrane, however diffusion also occurs where no barrier (or membrane) is present. A number of factors can affect the rate of diffusion, including temperature, molecular weight, concentration gradient, electrical charge, and distance.
Biology I Laboratory Manual. Optional Lab Activities. Search for: Osmosis and Diffusion. Lab Objectives. At the conclusion of the lab, the student should be able to: define the following terms: diffusion, osmosis, equilibrium, tonicity, turgor pressure, plasmolysis ... Diffusion can occur across a semipermeable membrane; however diffusion also ...