What is Human Tissue Testing?

When a drug leaves the lab-stage and is tested in animals, the response does not always match that seen in humans. Diseases such as cancer or diabetes can be very similar in animals, but there are differences. Human tissue research can help by looking at the effects of drugs in donated bits of tissue (e.g. skin, cancer biopsies, blood) to check possible side effects before testing in human beings. Hundreds of researchers across the globe are using human tissue research to improve the way drugs are tested and developed.

What is ex vivo research?
Ex vivo research is a broad area covering the use of whole tissues for estimating drug efficacy and safety. Human tissue testing uses human biospecimens that have been donated for research by patients. Human tissue tests are most commonly used is in drug discovery and development to make decisions about the safety and efficacy of drugs for human use using human systems. It has become a very common area in the past 10-15 years with the advent of precision medicine and the human genome project.
Interview with Dr David Bunton: David is CEO at REPROCELL Europe based in Glasgow, UK. He has a background in pharmacology and physiology and spun out an ex vivo research company called Biopta in 2002. Biopta was acquired by REPROCELL in 2015, and David now heads their European operations. To learn about the past, present, and future of human tissue testing, we interviewed Dr Bunton in this video. You can listen to the full interview here , or read on to find out more.
Where do human tissue samples come from?
The logistics for collecting biospecimens are key to the whole process. Human tissues are extremely precious samples donated by patients, so every project must start with gaining informed consent from tissue donors. There are three primary sources for human tissue samples:
1. Surgical residual material
During surgery, any tissue that is required for diagnostics is sent for further investigation. But if there is residual tissue left over, the pathologist will decide if it can be donated to research. The tissue will be preserved or stored in an appropriate way depending on the purpose of the research: if it's a static study where we're just looking at the tissue structure and not function, then the tissue could be frozen or fixed at that point in time. It's very important that the patient is informed. Usually, there's a patient information booklet that explains the uses and the journey that the tissue will go on and also whether it's for commercial or non-commercial research.
2. Transplant tissue
Transplantation is the primary objective with transplant organs, but if for any reason the organs may not be placed for transplant then they can be used for research.
3. Biopsy material
Biopsy material is where patients or volunteers donate samples. You will probably be familiar with the idea of blood donation, but skin and muscle biopsies can also be donated. Over the past year and a half, we've all become familiar with the use of nasal swabs to collect biological samples for COVID testing - these types of samples can also be used for research.

How long do human tissue samples survive in the lab and during transport?
For many ex vivo studies, tissue is used fresh to retain its physiological relevance. Often tissues are stored in a physiological buffer or culture media, and then transport to the laboratory as fast as possible so that functionality is maintained. How long the tissues survive during transportation really varies according to the tissue type, as different tissues have different metabolic rates and sensitivities to being out of the body. Heart tissue, for example, is very active and doesn't survive more than a few hours. Other tissues, such as skin, are less metabolically active so are transported and preserved a little easier. But typically, you have minutes to hours to get tissues into the lab and into a test system. It becomes a 24/7 lab operation to support these types of studies and the ex vivo companies that have grown in the past 10-15 years are experts in logistics. However, it is not just the science or the laboratory experiments, but the processes to collect, store, transport the tissue, and maintain it in good condition. It's key that the material you have is as close to the starting point in the body; you want it to represent that normal physiology or pathophysiology. In most experiments, tissues will also need qualified to determine whether they're in good enough condition to undergo the experimental protocol.
How willing are people to donate human tissue samples for research?
Patients are extremely supportive of ex vivo research, whether it's in the commercial or non-commercial sphere. A number of surveys and publications have looked at this area and have determined that over 95% of patients support this type of research. It is, therefore, critical to show the benefits of human tissue testing; show how medicines can be brought to market quicker, and how drug safety can be demonstrated more clearly. Precision medicine is one of the ways in which the public is becoming more aware of the need to understand, not only the responses in humans but how we individually respond to different drugs.

What research areas is human tissue testing used in?
The most common use of fresh tissues is in late-stage pre-clinical or lead optimization: where there's a small number of promising drug candidates, and the objective is to try and understand if data from cell-based models or animals translates to humans. Researchers may want to investigate whether they see the same signaling pathways activated, or if there are biomarkers present that indicate efficacy. Researchers may also want to investigate clinical problems: troubleshooting of clinical observations where a drug has not been tested sufficiently in human systems, and unexpected safety effects occur in the clinic. In this case, human fresh tissues can give mechanistic insights into those effects that may differ between humans and animals.
The most common application of human tissue testing is to make sure that animal data matches what is observed in humans before clinical trial. Human tissue testing uses complex intact tissues, but for pharmacology, you can achieve reasonably good throughput for this type of test. For example, 20 to 30 individual tissues can be obtained from a single donor, to look at multiple compounds.
What industries are using human tissue research?
The overwhelming use of human tissue testing is in pharma and biotech, but there have also been ex vivo studies in agrochemical and medical device areas. This type of research is often used where there is no suitable model in cells or animals that reflect what is being assessed. For example, assessing the risk of a particular chemical or pesticide on reproductive health, or recreating the physical characteristics of a blood vessel in the lab which may be important for certain medical devices such as stents.

What is the main need that human tissue research addresses?
Despite all our advances and improvements in science, there is still a problem with translation from pre-clinical to clinical — in particular, our prediction of efficacy. Human tissue testing can validate that a drug target will benefit patients, thereby reducing clinical attrition rates. Pharma can save huge sums of money by using ex vivo testing at these later stages where, unfortunately, failure is still common.
Depending on the therapeutic area, as many as one in five compounds can fail. Everyone knows that it's a problem, but it's a challenge that human tissue cannot address on it's own; there are too many factors involved. Instead, it's about reducing risk at each stage - not just through fresh tissues — but also through the use of stem cell models, complex 3D tissue models, and organ-on-a-chip technologies. These are all part of a solution that is helping to improve understanding of complex human biology.
What are the most common therapeutic areas investigated using human tissue samples?
Most ex vivo research originated in cancer - using tumor samples to characterize the differences between cancer types — which formed the foundation for a lot of tissue networks that remain a key area for drug discovery. Today, human tissue research covers many different types of therapies from cardiovascular disease to respiratory diseases e.g., asthma and COPD.
Over the past 10 years, the most rapidly growing field has been in autoimmune disorders such as psoriasis, atopic dermatitis, and inflammatory bowel disease. Crohn's disease and ulcerative colitis are on the increase, and there's a lot of focus in pharma on biologicals and preventing disease progression via early intervention. The key is to get the relevant tissue type fresh and use that as a model of drug effectiveness. That's one of the powers of the human tissue approach.

How does human tissue testing compare to other types of research?
There are there strengths and weaknesses to every approach, and human tissue testing is no different. Ex vivo research varies from traditional cell-based or animal approaches, in that it focuses less on reproducibility and robustness of the assay, and more on translation to humans. Responses in human tissues from 10 or 20 donors will be more variable than a comparative study in 20 rats that are inbred and therefore similar in their responses. This variability used to be seen as a weakness, but it's being increasingly understood as a strength - because variation in patient response is what happens during clinical trials and is a key reason why promising drugs sometimes fail. While they may be benefiting a section of the target patient population in the clinical study, other patients see little or no response.
Using human fresh tissue, you can understand inter-individual variation earlier in drug discovery, and therefore design clinical trials differently, and have more confidence that your target patient population is correct. Having a "fail fast, fail early" approach provides huge savings downstream because costs increase the closer you get to clinical trials. Having an increased understanding at the pre-clinical stage pays dividends when it comes to higher success in the clinic - even if it means more compounds are either discontinued or perhaps the target patient population is smaller than originally planned. I think it's unrealistic to say that human tissue testing could replace all animal experiments, but it certainly is already reducing and refining those experiments and there's going to be further progress over the next 10 years.

Can human tissue testing be performed in-house?
Companies usually outsource, as it requires specialist knowledge. There is a lot to consider before embarking on one of these projects, such as ethical approval, logistics of the supply chain, protocols, and validation of the different tissue types. That is a lot of variables by the time you factor in the different tissue types, targets, and platforms that can be used. So having groups that have got experience across a wide range of those things really helps.
Outsourcing human tissue testing to a commercial lab has a number of benefits. For example, regulatory approval is possible for safety assessments in human tissue, and having a quality assurance system is beneficial. It is also usually cheaper, as contract research organizations specializing in this type of research can make the process more economical,
Would you say that the demand for human tissue testing is increasing?
Yes, there's been an increase in the use of human tissue for research. It's been a trend that's been there the past 10-15 years, and will only increase through the drivers of precision medicine and artificial intelligence (AI).
How do you think the regulators view the data you generate with human tissue testing?
Although human tissue testing is not mandatory, the regulators are supportive of human tissue studies for safety pharmacology. They have gone through this process with companies and have tried to promote the appropriate use of human test systems. Around five years ago the FDA and MRHA surveyed the pharma industry about the barriers to increased use of human tissue testing for drug safety assessment. The study showed that most pharma companies were either using the technology or developing methods to use it, which included human fresh tissue and other approaches, such as 3D models.
The regulators are happy to take the additional data and, while it is not mandatory, it does reduce the amount of animal testing. Typically, if it's a target where it's understood that animal models may not reflect human biology (e.g, serotonin receptors) then the regulators may request that you need to include some human tissue systems. It could also be the sponsors in pharma themselves that identify potential risks and decide that they need human data.
What does the future hold for human tissue testing?
Right now, we have more data than anyone could imagine — the challenge is not generating that data but using it intelligently. Insights are not going to come from genomics or transcriptomics alone, because the predictions made just from looking at insights next-gen sequencing are not sufficient to understand pharmacology. To get to true pharmacogenomics, we need to include functional data and understand the other information about these individuals - clinical data, medical, and drug history. This is where AI could help us make breakthroughs because of the volume and complexity of this data. By appropriate use of AI machine learning models, researchers could achieve insights that wouldn't otherwise be possible.
The quality of the data you need must be very high
The quality of the data is key. A challenge is the number of donors that are needed, but a lot of effort is being made internationally, within both public and private sector initiatives, to safely share and gather data. For example, in Scotland, there is a network of "safe havens": National Health Service (NHS) run institutions that provide a firewall between the patient data in the healthcare system and pharmaceutical companies, or academic researchers. They ensure appropriate anonymization and access while still ensuring that researchers can make medical breakthroughs. Scotland-wide there is this health identification number that every individual has, from cradle to grave. That number identifies all of the health records and allows access to all medical and prescription history.
What exactly does REPROCELL do?
REPROCELL is a life science company that provides products and services - primarily in regenerative medicine and drug discovery — focused around human data, human tissue systems, and human-engineered tissue models.
We were one of the first companies to commercialize a particular type of stem cell called "induced pluripotent stem cells" which are taken from adult tissue and reprogrammed back into stem cells. It has been a very successful Japanese company in that field. REPROCELL has acquired a number of complementary businesses in the past five or six years around the stem cell and human tissue research space - all linked by a common theme of human data and human-relevant test systems.
Our group headquarters are in Yokohama in Japan, but our human tissue testing services are based in Glasgow. REPROCELL also has labs in Maryland, USA, and Hyderabad, India. It's a truly global business.
If any of our listeners would like to contact REPROCELL, how would they go about that?
We'd be delighted to discuss any of the ideas or concepts in the discussion today. The best way is through our website — we've got our contact details for all our sites and email addresses on there.

Editor's note: This blog was first published in October 2021 and has since been updated for accuracy and clarity.

Roland Linder
Senior Trade Officer at Scottish Development International Roland is based in Bern, Switzerland, and hosts a podcast called "What in the Life Sciences" which is is all about the life science industry. You can contact him on LinkedIn .
Subscribe to receive updates from REPROCELL
Subjects we write about
- 3D Cell Culture
- Cell Culture
- Central Lab Services
- Clinical Capabilities
- Disease Modeling
- Drug Discovery
- Gene Editing
- Genomic services
- Human Tissue Samples
- Human Tissue Testing
- Life Sciences
- Oligonucleotide Synthesis
- Precision Medicine
- Regenerative Medicine
- Respiratory Disease
- Safety Pharmacology
- Skin Disease
On the REPROCELL blog
Redefining Success in IBD Drug Discovery with Human Tissue Models
Redefining IBD drug discovery with a dual-pronged approach through human bioengineered and fresh tissue.
11 December 2024
Pharma AI is Changing Clinical Trials by Optimizing Patient Selection
AI is revolutionizing clinical trials by optimizing patient selection, improving trial outcomes, and accelerating the development of new drugs.
27 November 2024
Key Strategies Central Labs Use to Fast-Track Time to Market
Learn how central labs use automation, outsourcing, and AI to accelerate drug development, reduce costs, and improve clinical trial accuracy and efficiency.
18 November 2024
REPROCELL's Advanced Skin Tissue Engineering
Discover REPROCELL's pioneering 3D skin tissue engineering, enhancing drug development and skincare research with accurate and robust human skin models.
31 October 2024
Plasma or Serum? – You Decide.
Discover the key differences between plasma and serum, their collection methods, and their impact on clinical and experimental outcomes.
17 October 2024
Upcoming Events
Conferences we will be attending, and webinars hosted by us
Corporate News
PRESS RELEASE: New Study Highlights Role of Stromal Support in Bioengineered Human Intestinal Models
New study by Prof Stefan Przyborski at Durham University enhances drug testing models with stromal support in bioengineered human intestinal tissue.
13 November 2024
Press Release: Bioserve India Launches Advanced Stem Cell Products
Discover Bioserve India's advanced stem cell products, supporting innovation in research and drug development.
22 July 2024
REPROCELL USA Inaugurates GMP Manufacturing Facility for Stem Cells
REPROCELL USA unveils GMP manufacturing facility for hiPSC and hMSC, revolutionizing stem cell therapy with closed-system technology.
30 May 2024
All Gifts MATCHED!
Your gift matched dollar for dollar, up to $250,000! Deadline: December 31.
- For Clinicians
- For Medical Students
- For Scientists
- Our Victories
- Internships
- Annual & Financial Reports
- Barnard Medical Center
Access to Human Tissues
Human tissue models are biologically and physiologically relevant and can help to speed up research and development while saving money and reducing the number of animals used in science.
Human tissues are currently being used in many areas of biomedical research, including research on conditions and diseases like cancers, heart disease, diabetes, Alzheimer’s disease and other dementias, and for the treatment of conditions such as Parkinson’s and Multiple Sclerosis. It’s also common for human tissue to be used in microfluidic systems for toxicology testing and as biosensors to detect biological or chemical threat agents.
Locating Human Tissue for Research
There is no single organization or network that oversees the process of obtaining human tissue from consenting donors and providing it to researchers, but there are numerous organizations that can help.
Biorepositories
Biorepositories are libraries where biospecimens are stored and made available for clinical or research purposes. Around the globe there are hundreds of biorepositories, or biobanks. Many focus on collecting material from patients diagnosed with specific diseases, some are population-based, and others have an even broader focus.
Specimen Central is the global hub where biobanks and biomedical researchers meet to exchange human tissue needs and supplies. Specimen Central seeks to reduce delays and costs in research by expediting connections between demand and supply and by connecting biobanks, biorepositories, tissue archives, pathology labs, and reputable commercial human sample providers with qualified scientific researchers of all kinds.
Tissue Solutions is a virtual biobank that sources ethically acquired, fully consented tissue from its extensive global network. Tissue Solutions has a Volunteer Donor Database where anyone can register to donate samples for scientific research.
The International Society for Biological and Environmental Repositories (ISBER) oversees the International Repository Locator to help investigators locate biospecimen and data repositories by developing a directory of repository information that can be searched online.
The College of American Pathologists’ (CAP) Biorepository Accreditation Program and Laboratory Accreditation also offers a searchable database of CAP-accredited biorepositories.
The US Biolab is a global biobank that has access to a diverse range of human biospecimens in various disease states to meet researchers’ research needs.
The American Association of Tissue Banks (AATB) maintains a database of AATB accredited tissue banks in the U.S.
Organ Procurement Organizations and other tissue providers
Organ Procurement Organizations (OPOs) are non-profit organizations that are federally designated by the Center for Medicare and Medicaid Services. They are responsible for recovering organs from deceased donors for transplantation in the U.S. Many OPOs also work directly with researchers to provide donated human tissue for medical research, education, and testing. The Association of Organ Procurement Organizations (AOPO) maintains a list of OPOs by region . Many OPOs also work with researchers outside of their federally designated area.
In addition to OPOs, there are other tissue providers who specialize in providing human organs and tissues for medical research, education, and development. These providers can meet researchers’ specific requests, provide an array of tissue types from any body system, offer diseased and non-diseased biospecimens, and can arrange several preservation methods, including fresh, frozen, and fixed, suitable for various analytical techniques.
International Institute for the Advancement of Medicine works with OPOs, tissue banks, and donor families in the U.S. to provide biomaterials to qualified medical and scientific professionals around the world.
National Disease Research Interchange provides academic and corporate investigators worldwide with high-quality human biospecimens to advance biomedical research.
Tissue for Research works with biomedical companies and academic institutions to provide annotated human specimens for diagnostic, drug, or medical device research or for basic research. Tissue for Research has an inventory of biospecimens or can collect samples from most diagnoses to meet a researcher’s specifications.
Academic medical centers, hospitals, and surgical centers
Academic medical centers, hospitals, and surgical centers can also be a valuable resource for investigators in need of donated human tissue, including surgical remnants. Several medical centers in the U.S. have partnerships with universities and biotechnology companies to provide them with human tissue for research, treatment, and drug development purposes.
The Association of Academic Health Centers maintains a record of U.S. and international academic health centers. Researchers can contact centers to learn more about their practices in recovering and providing donate tissue for research.
Contact your local medical centers to learn more about their role in recovering tissue for research.

An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Use of human specimens in research: the evolving United States regulatory, policy, and scientific landscape
Marianna j bledsoe , ma, william e grizzle , md, phd.
- Author information
- Copyright and License information
The use of human specimens in research has contributed to significant scientific and medical advancements. However, the development of sophisticated whole genome and informatics technologies and the increase in specimen and data sharing have raised new questions about the identifiability of specimens and the protection of participants in human specimen research.
In the US, new regulations and policies are being considered to address these changes. This review discusses the current and proposed regulations as they apply to specimen research, as well as relevant policy discussions. It summarizes the ways that researchers and other stakeholders can provide their input to these discussions and policy development efforts. Input from all the stakeholders in specimen research will be essential for the development of policies that facilitate such research while at the same time protecting the rights and welfare of research participants.
Keywords: biorepositories, ethical issues, human specimen research, human subjects protection regulations, personalized medicine, research policy
Human specimens and human specimen biorepositories play a key role in scientific and medical advancement, and will continue to play a critical role in the future, particularly towards efforts to develop individualized medicine and targeted therapies. However, their widespread use raises a number of challenges, particularly in relation to the current regulatory, policy and scientific landscape. Discussed in this paper is the potential importance of human specimens and human specimen biorepositories to research and the current and evolving regulatory, policy and scientific landscape in the US as it applies to such research.
Introduction
Importance of human specimens in research.
The use of human specimens in biomedical research has been critical to the development of current medical care. Although animal cell lines and specimens are useful in most research, molecular features of animal specimens frequently are different from those of matching human specimens just as animal diseases are, in general, different from human diseases. For example, it was recently reported that mice are a poor model for studying the genetics of human inflammatory disease. 1 , 2 The importance of archival clinical specimens in support of biomedical advances has been elegantly described by Korn as the “intellectual foundation of modern medicine”. 3 Indeed, since this publication, significant additional advances in medical care have been based on research using human specimens. Included are many major advances that have not only changed therapies of diseases, but also have resulted in new concepts of human biology.
Therapeutic advances and approaches to medical care now are beginning to target unique molecular features of pathways that have been identified in human specimens as important to the development of specific diseases. The potential uses of molecular targeting have resulted in approaches to treat the unique features of an individual patient's disease (e.g., personalized or individualized medical care). These approaches will require even greater use of human specimens both in medical research and to aid in clinical decisions. 4 . 5 . 6 , 7
Specific targeting of features of molecular pathways has resulted in novel molecular directed therapies for once untreatable diseases, especially targeting specific molecules in different types of cancer. For example, the cellular surface receptor HER-2, was initially identified using archival human tissues and reported to be important in causing an aggressive subtype of breast cancer. Studies on human tissue led to the development of approaches to therapy that specifically target the HER-2 receptor. 8 Thus, a once poor prognostic feature of one type of breast cancer was changed by an effective strategy of molecular targeting to a good prognostic feature. 9 , 10 , 11 In addition, HER-2 targeted therapies are now being expanded to treat other forms of cancer, such as gastric cancer. 12
Recent advances using human tissue have expanded and changed our concepts of human cellular biology resulting in potential new approaches to the treatment of a wide variety of diseases. For example, it was discovered that some messenger RNAs (mRNAs), which are the precursor molecules that permit the production of all proteins, were regulated in human and other cells by a newly identified category of small molecule, microRNA. 13 Up regulation or down regulation of specific proteins may be involved in the development, progression, or severity of specific diseases; the level of these proteins frequently are affected by microRNAs. 13 , 14 Significantly, microRNAs are potential targets for specific therapies and the production of these proteins may be modulated by targeted microRNAs. 15 , 16 Several other paradigm-changing discoveries based on the use of human tissues also have altered our concepts of normal biology as well as causes of disease. 5 , 17 Given the demonstrated importance of human specimens to biomedical research and their role in developing approaches to targeted medicine, demand for human specimens has increased dramatically over the past several decades. 18 A number of types of human specimen biorepositories have been developed to help meet this demand.
Surgical Specimen Biorepositories
Because only a small proportion of tissues removed surgically are required for diagnosis, remnant tissues can be used to support biomedical research by constructing additional paraffin blocks for research or providing the remnant tissues as frozen and/or fresh viable tissue. The tissue required for diagnosis is embedded in paraffin and in addition to providing diagnostic information, also can be used in future research. Other innovative approaches to obtain samples for research may be applied to specimens that are too small or are in situ lesions; these include obtaining nitrocellulose blots as tissue aliquots. 19 These aliquots of residual surgical specimens may be stored for future research in various types of biorepositories.
The Cooperative Human Tissue Network (CHTN) is a prospective biorepository model in which tissues are collected specifically to meet investigator requests. 20 Alternatively, tissues can be banked for future use in research biorepositories following a standard operating procedure (SOP); such a banking model is typically utilized by Specialized Programs in Research Excellence (SPORE) 21 and the National Cancer Institute Clinical Cooperative Group Banks. 22 Each of these two models, prospective and banking, has advantages and disadvantages. The prospective model has the advantage of providing specimens which exactly meet an investigator's needs and is a model in which generally all specimens are utilized; however, neither specimens nor clinical outcomes are immediately available from prospective biorepositories because both must be collected over time. The banking model typically has multiple specimens immediately available as well as clinical outcomes for the specimens provided. The banking model is most appropriate for cases in which it is necessary to collect clinical or longitudinal data and the participants are being followed over time. The disadvantages to the banking model are that the SOPs that are used in collecting and processing specimens may not meet investigator needs and requirements, especially for use with future technologies that may not yet be developed, and that many specimens may never be used. The underutilization of specimens in biobanks has recently been proposed as an important ethical issue. 23 While both types of biorepositories are useful models, careful attention is needed in the design of the biorepository to ensure that specimens are optimally utilized. 6 , 17
While human specimens and human specimen biorepositories continue to be important to scientific and medical advances, the availability of large numbers of specimens and extensive associated demographic and clinical data, the powerful new genetic and genomic technologies such as whole genome sequencing and the enormous and rapid advances in informatics, raise a number of evolving ethical, legal and social issues related to the use of human specimens. Discussed below are the current US regulations and policies as they relate to specimen research, including human specimen biorepositories, and the evolving scientific and policy landscape in the US.
The Current US Regulatory, Policy and Scientific Landscape
Federal regulations that may apply to human specimen research.
In the US, there are three important federal regulations that may apply to the use of human tissue and associated data in research, the “Common Rule,” promulgated by the Department of Health and Human Services (HHS) and codified at Code of Federal Regulations (CFR) title 45 part 46, Subpart A 24 , the US Food and Drug Administration (FDA) human subjects regulations at 21 CFR part 50 25 , 56 26 , and 812 27 and the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule (45 CFR part 160 and Subparts A and E of part 164) 28 and Security Rule (45 CFR part 160 and Subparts A and C of part 164). 28 Each of these regulations is discussed in further detail below.
The Common Rule
The Common Rule has been codified by 15 US federal departments and agencies and applies to all research involving human subjects that is “conducted, supported or otherwise subject to regulation by any federal department or agency which takes appropriate administrative action to make this policy applicable to such research.” Each of these 15 federal department or agencies has a codification of the Common Rule which is equivalent to 45 CFR 46, Subpart A 1 . The Rule includes requirements for Institutional Review Board (IRB) review and informed consent for human subjects research.
The Common Rule defines “research” as a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge. Sometimes the definition is challenging to interpret as the difference between “research” and uses of patient specimens and associated data in education or clinically relevant activities becomes blurred. 29 Therefore, researchers and pathologists should consult their local IRB for guidance before beginning activities involving human specimens.
The Common Rule defines a human subject as a living individual about whom an investigator conducting research obtains either data through intervention or interaction with the individual; or identifiable private information [45 CFR 46.102(f)]. Therefore, the Common Rule would apply when specimens or associated information are obtained for research from a living individual through intervention or interaction with the individual, such as a blood draw or cheek swab, or when residual specimens taken during the course of routine care are collected prospectively for research purposes. It would also apply when identifiable specimens are used for research (i.e., when the identity of the subject is or may readily be ascertained by the investigator or associated with the specimens).
Furthermore, in order for research involving humans specimens to be considered human subjects research under the Common Rule, the individuals must be living. Thus, according to the Common Rule, as currently written, research involving material from deceased individuals (e.g. autopsy material) or the use of specimens that are completely anonymous (i.e. a link to subject identity does not exist), would not be subject to the Common Rule, although state and local regulations and policies may apply.
Under certain circumstances, research using coded specimens, that is, specimens for which identifying information has been replaced with a code, may not be considered human subjects research if certain conditions have been met 30 . The creation of a human specimen biorepository for research purposes is considered to be a research activity and would be considered to involve human subjects research if specimens and/or associated data are being collected through interaction or intervention with a living individual or if the human specimen repository includes the collection, distribution or use of identifiable private information.
For research using human specimens that is considered human subjects research, the Common Rule generally requires review by an Institutional Review Board (IRB) and informed consent from the subject/participant. However, research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens may be exempt from these requirements if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. [45 CFR 46.101(b)(4)].
Furthermore, the requirement for informed consent for use of human specimens may be waived by the IRB when all of the following conditions are met [45 CFR 46.116(d)]:
The research involves no more than minimal risk to the subjects;
The waiver or alteration will not adversely affect the rights and welfare of the subjects;
The research could not practicably be carried out without the waiver or alteration; and
Whenever appropriate, the subjects will be provided with additional pertinent information after participation. 2
It is important to note that these requirements are based on the Common Rule as currently written. As discussed later in this paper, changes to these requirements are under consideration. 31
The FDA Human Subjects Regulations
The second set of significant US federal regulations that may apply to the collection and use of human specimens for research are the Food and Drug Administration (FDA) regulations, 21 CFR 50 25 , 56 26 , and 812 27 . The FDA regulations apply to all clinical investigations regulated by the FDA under sections 505(i) and 520(g) of the Federal Food, Drug, and Cosmetic Act, as well as clinical investigations that support applications for research or marketing permits for products regulated by the FDA. Among other products included within the scope are drugs for human use, medical devices for human use and biological products for human use. 21 CFR 50 covers informed consent requirements and 21 CFR 56 covers IRB review requirements. 21 CFR 812.2(a) applies to all clinical investigations of devices to determine safety and effectiveness unless the device investigation is exempt under 812.2(c).
The FDA regulations define a human subject differently than the Common Rule. The FDA regulations at 21 CFR 50.3(g) and 56.102(e) define a human subject as an individual who is or becomes a participant in research, either as a recipient of the test article or as a control. A subject may be either a healthy human or a patient. (See 21 CFR 50.3(g) and 56.102(e)). The device regulations define a subject as an individual on whom or on whose specimen an investigational device is used. (See 21 CFR part 812).
Unlike the Common Rule, the FDA regulations do not require the research participant/subject to be identifiable for the regulations to apply. Furthermore, unlike the Common Rule, the FDA exemptions to the requirement for informed consent are limited to emergency, life threatening situations, and military operations. This may pose challenges for some studies involving human specimens (e.g. the development of assays using archived specimens when it is difficult or impossible to contact the individual to obtain informed consent). In order to address this issue, the FDA issued guidance stipulating that the FDA would exercise enforcement discretion with regard to requiring informed consent when leftover human specimens that are not individually identifiable are used in FDA-regulated in-vitro diagnostic investigations, if certain conditions specified in the guidance are met. 32
The Health Insurance Portability and Accountability Act, Privacy and Security Rules
The third major set of US federal regulations that may be relevant to some human specimen research is the Health Insurance Portability and Accountability Act (HIPAA) Privacy and Security Rules. 28 The Privacy Rule regulates the uses and disclosures of individually identifiable health information by “covered entities” (health care providers, health plans, and health care clearinghouses). While the Privacy Rule does not apply to the use of human specimens per se, it may apply to uses and disclosures of the health information that may be associated with the specimens. The Privacy Rule generally requires patient authorization for uses and disclosures of health information that is individually identifiable. [See 45 CFR part 164.508]. Authorization is a similar but not identical concept to informed consent. Informed consent is the process by which subjects are informed about the risks and benefits of participating in research whereas authorization is solely a permission to allow researchers to use or disclose defined protected health information.
The ways in which protected health information may be used and disclosed for research is summarized in Table 1 . Patient authorization is not required if the information to be used or disclosed is de-identified according to the Privacy Rule's requirements at Section 164.514 (See Table 2 ) or a “Limited Data Set” (See Table 3 ) pursuant to a Data Use Agreement that meets the requirements of the Rule [See 45 CFR 164.514(e)]. Patient authorization for the uses and disclosures of protected health information is also not required if an IRB has waived the requirement for authorization according to criteria stipulated in the Rule, for purposes “preparatory to research” or for research solely on decedents if certain representations are made to the IRB. The US Department of Health and Human Services has provided additional guidance on how the Privacy Rule applies to research 33 , as well as guidance on de-identification. 34 The HIPAA Security Rule establishes national standards to protect individuals' electronic personal health information that is created, received, used, or maintained by covered entities. 28 The Security Rule (45 CFR Part 160 and Subparts A and C of Part 164) includes requirements for appropriate administrative, physical and technical safeguards to ensure the confidentiality, integrity, and security of electronic protected health information. These requirements may apply to research databases, such as those that may be associated with specimen collections, including those of individual investigators.
Table 1. Ways in Which Protected Health Information Can be Used and Disclosed by Covered Entities For Research Under the HIPAA Privacy Rule [45 CFR parts 160, 162, and 164].
Table 2. de-identified data set 3 4 ..
Excerpt taken from “Protecting Personal Health Information in Research: Understanding the HIPAA Privacy Rule”. U.S. Department of Health and Human Services.
Note Covered Entities may also use statistical methods to establish de-identification instead of removing all 18 identifiers, [See 45 CFR part 164.514(b)].
Table 3. Limited Dataset Under the Privacy Rule 1 .
Other applicable regulations and policies.
In addition to the aforementioned US federal regulations, there may be state and local regulations or funding agency policies that may apply to human specimen research. For example, some states have their own human subjects regulations (e.g. New York, Maryland, Virginia, and California). 35 In addition, state laws concerning genetic testing, genetic or medical record privacy also may apply and these may vary considerably from state to state. 35 Certain human specimen research funded by the National Institutes of Health (NIH) may be subject to resource and data sharing policies, such as the NIH policy on genome-wide association studies. This policy calls for investigators funded by the NIH for genome-wide association studies to share de-identified genotypic and phenotypic data through a centralized NIH data repository. 36
In other countries, there may be different ethical and privacy regulations and policies that may apply to the use of human specimens and associated data in research. 37 . These regulations and policies, especially in the European Union, are evolving rapidly and need to be considered when international collaborations are involved in research involving human specimens and/or data.
The Evolving Legal and Ethical Landscape Related to Human Specimen Research
Some of the aforementioned US regulations governing human subjects research were written a decade or two ago. Since that time, the research environment has evolved dramatically from research conducted in single laboratories to national and international multi-site collaborations between academia, government, industry and non-profit entities. Specimen and data sharing has also increased significantly, with many funding agencies now expecting broad sharing of research tools and data. Furthermore, the advent of affordable whole genome technologies, the increase in research databases, and implementation of electronic health records have raised new questions about privacy. At the same time, advances in technology are raising new questions regarding the identifiability of specimens and genomic data.
A number of cases in the media, both in the US and abroad have underscored some of the ethical, legal and social issues related to the use of human specimens in research. The Alder Hey organs scandal in the UK, involved the unauthorized removal, retention, and disposal of human tissue, including children's organs, from 1988 to 1995, and led to the Human Tissue Act 2004, and the creation of the Human Tissue Authority. 38 , 39 A number of cases in the US, have also highlighted important ethical issues related to the use of human specimens for research. Issues related to informed consent and the commercial use of tissue were highlighted in a recent best seller 40 concerning Henrietta Lacks, the daughter of a poor African- American tobacco farmer whose specimens were obtained without her knowledge or consent and used to develop cell lines which have been shared broadly and sold throughout the world. More recently, the posting of Henrietta Lacks' genomic sequence on a publicly available website without the consent of her family led to its removal. 41 , 42 The retention of blood spots obtained from newborn children for research without parental consent led to a lawsuit in Texas resulting in the destruction of approximately 5 million samples. 43 , 44 Other cases such as the Moore Case 45 , Canavan Case 46 , and Catalona Case 47 , 48 , 49 involved lawsuits regarding claims of private ownership of human specimens used in research. In none of these cases did courts find that research participants had any ownership rights to their tissue, although the courts noted the importance of informed consent. 50 To date, there is no federal law addressing the ownership of human tissue.
In another case that received attention in the US, specimens were collected from members of the Havasupai tribe for research on diabetes. The specimens were later used for studies of migration and other purposes that the tribe found objectionable. Tribal members sued the investigators and the university claiming fraud, breach of fiduciary duty, intentional infliction of emotional distress, negligence, conversion and lack of informed consent. The lawsuit also alleged that the researchers allowed wholesale transfer of blood samples from laboratory to laboratory and university to university and that many samples could not be accounted for. The lawsuit was settled with a payment of $700,000 to the tribe along with the return of the blood samples, any derivatives, and associated data and documentation. 51 , 52
These cases demonstrate the importance of informed consent and transparency in specimen research. They also demonstrate the need to respect cultural perspectives in the conduct of such research, and the importance of having systems in place for tracking specimens when they are distributed for additional research and mechanisms for ensuring specimens are used consistent with informed consent.
These cases also illustrate the need for sound governance mechanisms and best practices for the collection, storage, distribution and use of human specimens in research. A number of best practices have been developed in this area. These include the International Society for Biological and Environmental Repositories Best Practices 53 , the National Cancer Institute (NCI) Best Practices 54 , and the Organisation for Economic Cooperation and Development (OECD) Guidelines for Human Biobanks and Genetic Research Databases. 55
In the US, new regulations and policies are being considered to address the changes in the research environment. 56 The changes in the regulations being contemplated were discussed in an Advanced Notice of Proposed Rulemaking (ANPRM) entitled “Enhancing Protections for Research Subjects and Reducing Burden, Delay, and Ambiguity for Investigators” published on July 26, 2011. 31 The ANPRM discusses a number of changes to the Common Rule that are being contemplated to provide additional protections for participants of research, as well as reforms to reduce the burden to the research community.
The ANPRM addressed a number of issues related to human specimen research and invited comments on them. Among the issues for which public comments were solicited is whether specimens should in themselves be considered identifiable, whether consent should be required for unidentified specimens, and whether a broad (non-specific) consent for future use of tissues should be considered acceptable. In addition, a new category of research was discussed, an “excused” category of research involving secondary use of specimens and identifiable information in which consent is required but there is no IRB review, unless the researcher plans to contact subjects with individual research results. Issues related to the use of human specimens discussed in the ANPRM are summarized in Table 4 . During the public comment period of 90 days, more than 1,100 comments were received in response to the proposed Rule.
Specimen-Related Issues on Which Comments Were Solicited in the Advanced Notice of Proposed Rulemaking, “Human subjects research protections: enhancing protections for research subjects and reducing burden, delay, and ambiguity for investigators” 5 .
Federal Register. July 26, 2011. 76 CFR 44512. HHS-OPHS-2011-005.
A Notice of Proposed Rulemaking is only one, preliminary step in the regulation making process. The Administrative Procedure Act, Pub.L. 79-404, 60 Stat. 237, governs the way federal agencies may propose and establish regulations. This Act generally requires agencies to publish all proposed new regulations in the Federal Register at least 30 days before they take effect and provide a way for the public to comment on the proposed regulation. The agency can then decide whether to proceed with the rulemaking process and if so, incorporate the public comments into a Notice of Proposed Rulemaking. This Notice of Proposed Rulemaking is issued for public comment before finalizing and publishing a Final Rule. However, an agency may decide to take no further action at any step of this process. At the time of the writing of this article, a Notice of Proposed Rulemaking on the proposed changes to the Common Rule has not been issued.
The scientific and policy landscape is evolving to reflect new ethical issues and privacy challenges related to advancements in science and technology. Recent studies demonstrating the potential to identify individuals by their genomic data, even when stripped of traditional identifiers has raised new questions about how best to protect participants who contribute their specimens to research. Homer and colleagues demonstrated that they could detect an individual's SNP profile in a mixture of DNA from 1,000 individuals. 57 This led to a change in NIH's data sharing policies for whole genome association studies to provide further protection of aggregate genome wide association study data shared through the Database of Genonotypes and Phenotypes (dbGaP). 58 , 59 In another more recent study, researchers were able to identify anonymous DNA donors in the 1,000 Genomes Project by matching their DNA sequences to publicly available genealogy databases. 60
Another area of considerable discussion related to human specimen research is when individual research results should be provided to research participants. The issue of when individual research results should be returned to research participants is not addressed explicitly in US federal regulations.
From an ethical perspective, the issue of return of results and incidental findings has been debated for many years, and a number of groups have made recommendations in this area. 61 , 62 Arguments for return of results include respect for persons, beneficence, reciprocity, justice, and the duty to rescue.
Arguments against return of research results include the view that the original intent is an altruistic donation to help research, that return of research results would promote a therapeutic misconception, and perhaps most importantly, that harms can accrue when individual research findings that are incorrect or have not been validated are returned to participants or their physicians.
More recent discussions have focused on when and how research results and incidental findings should be returned to individuals from genomic biobanks. 63 However, the return of research results from genomic biobanks is complex, with not only ethical implications, but legal and practical implications; thus considerable caution in the return of such findings is needed. 64 , 65
The issues raised by the advent of genomic technologies are being explored by the Presidential Commission for the Study of Bioethical Issues, a panel of experts who advises the President on bioethical issues arising from advances in biomedicine and related areas of science and technology. The Commission recently issued a report entitled, “Privacy and Progress in Whole Genome Sequencing”. 66 The Commission recommended strong baseline protections for whole genome sequence data and urged federal and state governments to ensure a consistent floor of individual privacy protections covering whole genome sequence data across state lines. They also recommended that clinicians and researchers use robust and understandable informed consent procedures when conducting whole genome sequencing and that the federal government facilitate broad public access to the important clinical advances that result from whole genome sequencing. As its next project, the Commission has taken up the return of incidental findings, including those arising during the course of genomic research and other research on human specimens.
While the Commission's recommendations do not constitute official policy guidance, these discussions and other policy development efforts may have a significant impact on biorepositories and the use of human specimens in research and should be followed closely by the research community.
Summary and Conclusions
As the scientific and policy landscape continues to evolve in the US, it will be important for researchers and other stakeholders to provide input as new regulations and policies are developed. Researchers, research participants, and other relevant stakeholders can follow publication of regulations and policies in the Federal Register 67 and Regulations.gov 68 and provide comments through Regulations.gov during the public comment period. Additionally, the International Society for Biological and Environmental Repositories (ISBER) 69 Science Policy Committee tracks policy and regulatory developments in the US and abroad, disseminates information to ISBER members and provides comments on behalf of ISBER. Nonetheless, comments from individuals as well as groups representing them are also important. Active engagement of all the relevant stakeholders will be essential to help inform the development of policies related to the use of specimens in research that will allow important research to proceed, while at the same time protecting participants of such research, their privacy and the confidentiality of their data. Responsible stewardship of specimens used for research will be critical to ensure that public trust is maintained in the research enterprise.
Acknowledgments
The authors would like to thank Rina Hakimian for her helpful comments on this manuscript. This article was supported in part by the following grants to W.E.G.: NIH/NCI 5 U01 CA 44968-23; NIH P30 CA 13148-40; P30 AR 50948-07; NIH CA 089019-10; NIH P50 CA 101955-07; NIH/NCI 2 U54 CA 118948-06; DOD W81XWH-10-1-0543 and the Center for Tissue Processing to Support Research in Pulmonary Hypertension - CMREF (Grant Number N/A).
Although they have not issued the Common Rule in regulations, three other agencies comply with the Rule. For a complete list of agencies that follow the Common Rule, see http://www.hhs.gov/ohrp/humansubjects/commonrule/ .
This requirement was originally intended to apply to ‘deception research,’and generally believed to rarely, if ever, apply to research on human specimens.
Authors' Disclaimer: The viewpoints expressed herein represent the authors' personal perspectives and not that of any affiliated institution or organization. The contents of this review do not constitute legal advice or official policy guidance. Researchers should consult their Institutional Review and Privacy Boards, and other relevant institutional officials for local legal, regulatory and policy interpretations, as well as other local requirements for their work.
Conflicts of interest: M.J.B. is a member of the International Society for Biological and Environmental Repositories (ISBER).
Conflicts of interest: W.E.G. operates tumor banks as part of the Breast, Pancreatic and Cervical SPOREs at the University of Alabama at Birmingham and the Pulmonary Hypertension Breakthrough Initiative and prospective tissue repositories as part of the Cooperative Human Tissue Network and the Comprehensive Cancer Center and is a member of ISBER. He is also a member of the ethics group of the U54 grant, Morehouse School of Medicine/Tuskegee University/University of Alabama at Birmingham Comprehensive Cancer Center Partnership.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marianna J. Bledsoe, Silver Spring, MD, USA.
William E. Grizzle, Division of Anatomic Pathology, Department of Pathology, The University of Alabama at Birmingham, Birmingham, AL, USA.
- 1. Seok J, Warren HS, Cuenca AG, et al. Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–12. doi: 10.1073/pnas.1222878110. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Grizzle WE, Moore HM. Mice model findings and the future of human tissue in disease research. Biopreservation and Biobanking. 2013:111–2. doi: 10.1089/bio..2013.1132. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Korn D. Contribution of the human tissue archive to the advancement of medical knowledge and the public health. Research involving human biological materials: ethical issues and policy guidance. [Accessed 25 May 2013];National Bioethics Advisory Commission. 2000 2:E1–E30. Available for download at http://bioethics.georgetown.edu/nbac/pubs.html . [ Google Scholar ]
- 4. Grizzle WE, Srivastava S, Manne U. Translational Pathology of Neoplasia. In: Srivastava S, Grizzle WE, editors. Translational Pathology of Early Cancer. IOS Press BV; Amsterdam, The Netherlands: 2012. pp. 7–20. [ Google Scholar ]
- 5. Grizzle WE, Srivastava S, Manne U. The biology of incipient, pre-invasive or intraepithelial neoplasia. In: Srivastava S, Grizzle WE, editors. Translational Pathology of Early Cancer. IOS Press BV; Amsterdam, The Netherlands: 2012. pp. 21–39. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Grizzle WE, Bell WC, Sexton KC. Issues in collecting, processing and storing human tissues and associated information to support biomedical research. In: Srivastavas S, Grizzle WE, editors. Translational Pathology of Early Cancer. IOS Press BV; Amsterdam, The Netherlands: 2012. pp. 531–549. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Srivastava S, Grizzle WE. Biomarkers and the genetics of early neoplastic lesions. In: Srivastava S, Grizzle WE, editors. Translational Pathology of Early Cancer. IOS Press BV; Amsterdam, The Netherlands: 2012. pp. 41–64. [ Google Scholar ]
- 8. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Jackish C. HER-2-positive metastatic breast cancer: optimizing trastuzumab-based therapy. The Oncologist. 2006;11(1):34–41. doi: 10.1634/theoncologist.11-90001-34. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26:3637–3643. doi: 10.1038/sj.onc.1210379. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Von Minckwitz G, Schwedler K, Schmidt M, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011;4:2273–2281. doi: 10.1016/j.ejca.2011.06.021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Ruschoff J, Dietel M, Baretton G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457(3):299–307. doi: 10.1007/s00428-010-0952-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. McNally LR, Manne U, Grizzle WE. Post-transcriptional processing of genetic information and its relation to cancer. Biotech Histochem. doi: 10.3109/10520295.2012.730152. in press. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Volinia S, Calin GA, Chang-Gong L, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. PNAS. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Krützfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438 doi: 10.1038/nature04303. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Wu Y, Crawford M, Mao Y, et al. Therapeutic delivery of MicroRNA-29b by cationic lipoplexes for lung cancer. Mol Ther Nucleic Acids. 2013:2–e84. doi: 10.1038/mtna.2013.14. PMID: 23591808. PMCID: PMC3650246. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Grizzle WE, Sexton KC, Bell WC. Human Tissue Biorepository. In: Cheng L, Zhang D, Eble J, editors. Molecular Genetic Pathology. 2nd. Vol. 1. Springer Science+Business Media; 2013. pp. 483–497. [ Google Scholar ]
- 18. Hughes SE, Barnes RO, Watson PH. Biospecimen use in cancer research over two decades. Biopreservation and Biobanking. 2010;8(2):89–97. doi: 10.1089/bio.2010.0005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Gaston SM, Soares MA, Siddiqui MM, et al. Tissue-print and print-phoresis as platform technologies for the molecular analysis of human surgical specimens: Mapping tumor invasion of the prostate capsule. Nat Med. 2005;11(1):95–101. doi: 10.1038/nm1169. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. National Cancer Institute CHTN Cooperative Human Tissue Network. [Accessed 25 May 2013]; http://www.chtn.nci.nih.gov .
- 21. National Cancer Institute Translational Research Program. [Accessed 25 May 2013]; http://trp.cancer.gov .
- 22. National Cancer Institute (NCI) Cooperative Group Banks: Biorepositories for NCI-sponsored Clinical Trials. [Accessed 25 May 2013]; http://cgb.cancer.gov .
- 23. Cadigan RJ, Dragana L, Haldeman K, et al. Neglected ethical issues in biobank management: Results from a U.S. study. Life Sciences, Society and Policy. 2013;9:1. doi: 10.1186/2195-7819-9-1. published on-line March 2013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Code of Federal Regulations Title 45 Public Welfare Department of Health and Human Services Part 45 Protection of Human Subjects. [Accessed 25 May 2013]; http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html .
- 25. Food and Drugs. Food and Drug Administration Department of Health and Human Services; [Accessed 25 May 2013]. Code of Federal Regulations Title 21. Chapter I. Subchapter A. General Part 50. Protection of Human Subjects http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=50&showFR=1 . [ Google Scholar ]
- 26. Food and Drugs. Food and Drug Administration Department of Health and Human Services; [Accessed 25 May 2013]. Code of Federal Regulations Title 21. Chapter I. Subchapter A. General Part 56. Institutional Review Boards. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=56 . [ Google Scholar ]
- 27. Food and Drugs. Food and Drug Administration Department of Health and Human Services; [Accessed 25 May 2013]. Code of Federal Regulations Title 21. Chapter I. Subchapter H. Medical Devices Part 812 Investigational Device Exemptions. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=812 . [ Google Scholar ]
- 28. U. S. Department of Health and Human Services. Combined Text of All Rules. [Accessed 17 June 2013]; [45 CFR Parts 160, 162, and 164]. Available for download at: http://www.hhs.gov/ocr/privacy/hipaa/administrative/combined/index.html .
- 29. Bell WC, Sexton KC, Grizzle WE. How to efficiently obtain human tissues to support specific biomedical research projects. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1676–9. doi: 10.1158/1055-9965.EPI-08-0820. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. OHRP Guidance on Research Involving Coded Private Information or Biological Specimens. [Accessed 25 May 2013]; http://www.hhs.gov/ohrp/humansubjects/guidance/cdebiol.pdf .
- 31. Human subjects research protections: enhancing protections for research subjects and reducing burden, delay, and ambiguity for investigators. Federal Register. 2011 Jul 26; 76 CFR 44512. HHS-OPHS-2011 005. [ Google Scholar ]
- 32. FDA. Guidance on Informed Consent for In Vitro Diagnostic Device Studies Using Leftover Human Specimens that are Not Individually Identifiable. [Accessed 25 May 2013];:34. http://www.fda.gov/cdrh/oivd/guidance/1588.html .
- 33. [Accessed 25 May 2013];U S Department of Health and Human Services Protecting Personal Health Information in Research: Understanding the HIPAA Privacy Rule. Available for download at http://privacyruleandresearch.nih.gov/pr_02.asp .
- 34. [Accessed 25 May 2013];U S Department of Health and Human Services Guidance Regarding Methods for De-identification of Protected Health Information in Accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/De-identification/guidance.html .
- 35. Hakimian R, Taube S, Bledsoe M, Aamodt R. National Cancer Institute Cancer Diagnosis Program: 50-State Survey of Laws Regarding the Collection, Storage, and Use of Human Tissue Specimens and Associated Data for Research. Bethesda, MD: National Institutes of Health; 2004. [Accessed 25 May 2013]. NIH publication 05 5628. http://cdp.cancer.gov/humanSpecimens/survey/index.htm . [ Google Scholar ]
- 36. U. S. Department of Health and Human Services, National Institutes of Health. [Accessed on 25 May 2013];Genome-Wide Association Studies (GWAS) http://gwas.nih.gov/
- 37. U.S. Department of Health and Human Services Office for Human Research Protections. [Accessed on 25 May 2013];International Compilation of Human Research Standards. http://www.hhs.gov/ohrp/international/intlcompilation/intlcompilation.html .
- 38. The Royal Liverpool Children's Inquiry: Summary and Recommendations. [Accessed 25 May 2013];The House of Commons. 2001 Jan 30; http://www.official-documents.gov.uk/document/hc0001/hc00/0012/0012_i.pdf .
- 39. The National Archives. Legislation.gov.uk. Human Tissue Act 2004. [Accessed 25 May 2013]; http://www.legislation.gov.uk/ukpga/2004/30/contents .
- 40. Skloot R. The Immortal Life Of Henrietta Lacks. New York: Crown Publishing, Random House, Inc.; 2010. [ Google Scholar ]
- 41. Skloot R. The Immortal Life of Henrietta Lacks, the Sequel. New York Times. 2013 Mar 23; [ Google Scholar ]
- 42. Travis J. Privacy Flap Forces Withdrawal of DNA Data on Cancer Cell Line. Science Insider 2013. 2013 Mar 26; [ Google Scholar ]
- 43. Grody WW, Howell RR. The fate of newborn screening blood spots. Pediatric Research. 2010;67(3):237. doi: 10.1203/PDR.0b013e3181d00a48. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Fikac P. State to destroy newborns' blood samples. [Accessed 25 May 2013];The Houston Chronicle. 2009 Dec 22; http://www.chron.com/news/houston-texas/article/State-to-destroy-newborns-blood-samples-1599212.php .
- 45. Moore v Regents of the University of California. 793P2d479. (Cal Rptr 146 1990) [ Google Scholar ]
- 46. Greenberg v Miami Children's Hospital Research Institute. 264(F Supp 2d):1064. (SD Fla 2003) [ PubMed ] [ Google Scholar ]
- 47. Washington University v Catalona. 437(FSupp2d):985. (ED Mo 2006) [ Google Scholar ]
- 48. Washington University v Catalona. 400F3d:667. (8thCir 2007) [ Google Scholar ]
- 49. Kaiser J. Court decides tissue samples belong to university, not patients. Science. 2006;312:346. doi: 10.1126/science.312.5772.346. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Hakimian R, Korn D. Ownership and use of tissue specimens for research. JAMA. 2004;292:2500–2505. doi: 10.1001/jama.292.20.2500. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Kiefer M. Havasupai Tribe ends regents lawsuit with burial. [Accessed on 25 May 2013];The Arizona Republic. 2010 Apr 22; Available for download at http://www.azcentral.com/arizonarepublic/local/articles/2010/04/22/20100422arizona-havasupai-tribe-regents-lawsuit.html .
- 52. Mello MM, Wolf LE. The Havasupai Indian tribe case: lessons for research involving stored biologic samples. N Engl J Med. 2010;363:204–207. doi: 10.1056/NEJMp1005203. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Campbell L, Betsou F, Garcia DL, Giri JG, Pitt KE, Pugh RS, et al. ISBER 2012 Best Practices for Repositories: Collection, storage, retrieval and distribution of biological materials for research. Biopreservation and Biobanking. (3rd) 2012;10:79–161. doi: 10.1089/bio.2012.1025. Available for download at http://www.isber.org/?page=BPR . [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. National Cancer Institute 2011 Best Practices for Biospecimen Resources. [Accessed on 25 May 2013]; Available for download at http://biospecimens.cancer.gov/practices/
- 55. Organisation for Economic Cooperation and Development (OECD) Guidelines for Human Biobanks and Genetic Research Databases. [Accessed on 25 May 2013];2009 Available for download at http://www.oecd.org/sti/biotech/guidelinesforhumanbiobanksandgeneticresearchdatabaseshbgrds.htm .
- 56. Emanuel EJ, Menikoff J. Reforming the regulations governing research with human subjects. NEJM. 2011;365(12):1145–1150. doi: 10.1056/NEJMsb1106942. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Homer N, Szelinger S, Redman M, et al. Resolving individuals contributing trace amounts of DNA to highly complex mixtures using high-density SNP Genotyping Microarrays. PLOS Genetics. 2008 Aug 29; doi: 10.1371/journal.pgen.1000167. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. NCBI Database of Genotypes and Phenotypes. [Accessed 25 May 2013]; http://www.ncbi.nlm.nih.gov/gap .
- 59. Zerhouni EA, Nabel EG. Protecting aggregate genomic data. Science. 2008 Oct 3;322(5898):44. doi: 10.1126/science.322.5898.44b. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Gymrek M, McGuire AL, Golan D, Halperin E, Erlich Y. Identifying Personal Genomes by Surname Inference. Science. 2013;339(6117):321–324. doi: 10.1126/science.1229566. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Wolf SM, Lawrenz FP, Nelson CA, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics. 2008;36:219–248. doi: 10.1111/j.1748-720X.2008.00266.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Fabsitz RR, McGuire A, Sharp RR, et al. National Heart, Lung and Blood Institute working group: Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung and Blood Institute working group. Cir Cardiovasc Genet. 2010;3:574–580. doi: 10.1161/CIRCGENETICS.110.958827. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Wolf SM, Crock BN, Van Ness B, et al. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genetics in Medicine. 2012;14:361–384. doi: 10.1038/gim.2012.23. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Bledsoe MJ, Grizzle WE, Clark BJ, Zeps N. Practical implementation issues and challenges for biobanks in the return of individual research results. Genetics in Medicine. 2012;14:478–483. doi: 10.1038/gim.2011.67. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Bledsoe MJ, Clayton EW, McGuire AL, Grizzle WE, O'Rourke PP, Zeps N. Return of research results from genomic biobanks: cost matters. Genetics in Medicine. 2013;15:103–105. doi: 10.1038/gim.2012.105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. The Presidential Commission for the Study of Bioethical Issues. [Accessed on 25 May 2013];Privacy and Progress in Whole Genome Sequencing. 2012 Oct; Available for download at http://bioethics.gov/node/764 .
- 67. National Archives and Records Administration. [Accessed on 27 May 2013];The Federal Register. https://www.federalregister.gov/
- 68. Regulations.gov. [Accessed on 27 May 2013]; http://www.regulations.gov/#!home .
- 69. The International Society for Biological and Environmental Repositories. [Accessed on 17 June 2013]; http://www.isber.org/
- View on publisher site
- PDF (170.8 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Frontiers for Young Minds
- Download PDF
Who Can Use Your Cells Or Tissues For Scientific Research?

Informed consent is the process of obtaining permission from human participants to use their cells and tissues or otherwise include them in research studies. With informed consent, scientists can use human cells or tissues in experiments to learn more about the human body and to test new medicines. This article describes how these tissues are obtained, and the ethical concerns regarding the use of human tissues in research. The story of Henrietta Lacks and her immortal HeLa cell line is discussed, to demonstrate the importance of informed consent and to showcase Henrietta’s valuable contributions to research and modern medicine.
What is Human Tissue Research?
Scientists can study cells or tissues from the human body in a lab to learn more about how the body works. These tissues can be blood, parts of organs, or cells. Tissues can be collected from living or dead patients, depending on the organ that is being studied [ 1 ]. Tissue research requires informed consent from the patient—basically the patient needs to understand what will be studied and say it is OK to use their tissues. Informed consent for tissue research must be obtained without coercion : coercion is a way of persuasion, usually with a threat or force [ 2 ]. Without informed consent from the patient, tissues cannot be used in any type of research. Tissue research has provided many advances in human medicine [ 2 ]. It is important to understand where tissues come from, how patients are involved, and the scientific advancements that have been made from human tissue research.
Why Do We Use Human Tissues in Scientific Research?
An in vitro experiment is one that is conducted with cells or tissues from the body, but the experiment is done outside of the body, such as in a test tube or culture dish. Scientists conduct in vitro research because research with cells and tissues allows them to perform experiments they could not perform on humans [ 1 ]. These experiments can use test drugs or toxic chemicals that might harm living participants, but because tissues are removed from the body, researchers can use them to learn more about how drugs or chemicals might affect a person.
How are Human Tissues Collected for Research?
Scientists can collect donated tissues that are left over from medical procedures such as surgeries, or from people who agree to donate their tissues for research after their death. These samples would otherwise be discarded if they were not used for research. Once the sample is collected, it is quickly transported to a lab and preserved at very cold temperatures, or experiments are performed right away with fresh samples. It is important for researchers to preserve donated tissues quickly since cells and tissues can break down outside the human body, which may alter the results of experiments. Research companies may also collect and store donated tissues that scientists can then purchase for their own research.
How Do Researchers Protect the Rights of Human Tissue Donors?
There are several important ethical concerns in using human cells and tissues for research. These include obtaining permission from donors and protecting their privacy. Fortunately, there are laws and guidelines in place to ensure that the rights of donors are protected.
In the United States, the Health Insurance Portability and Accountability Act (HIPAA) is a law that protects the privacy of donors by requiring samples to be de-identified after they are donated [ 3 ]. This means that samples are labeled so that researchers do not know the identity or personal health information of the donors [ 3 ]. Instead, researchers may only know basic information such as the age, sex, and race of the donor [ 3 ].
In addition, samples are collected only with the permission of an Institutional Review Board (IRB) at the location where the samples are collected [ 4 ]. The IRB makes sure donors are adequately informed about how and why their samples will be collected. The IRB also ensures that donors give informed consent to researchers before their tissues are collected. During the informed consent process, participants speak directly with research study staff, and they are informed of any potential risks or benefits associated with the research. Participants are encouraged to ask questions about their role in the research before choosing whether to donate their tissues. Participation in the research is completely the participant’s choice. Throughout the study, participants may continue to ask questions and can choose to leave the study at any time without a penalty. Regardless of whether a patient decides to participate in a study or donate their tissues for research, the quality of their healthcare will not be affected. Figure 1 shows the process of how human tissues are collected and used for research.
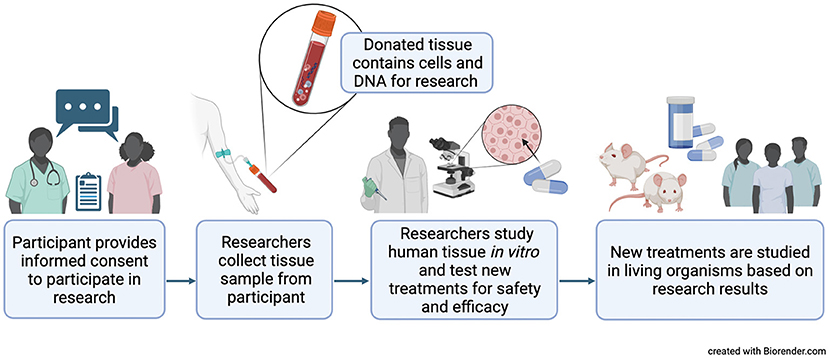
- Figure 1 - Research on cells and tissues outside of a living organism, called in vitro research, allows scientists to perform experiments they could not perform on humans, like testing new medicines for safety.
- Doctors and scientists must obtain informed consent from donors before they take any tissues for research use. This means that the donors must be informed about how their samples will be used, and they have the right to say no if they do not want to participate. Created with BioRender.com .
Over the years, there has been disagreement about how humans and their cells can be used for research. In 1964, the World Medical Association published the Helsinki Declaration, which made rules to protect humans in research all over the world [ 5 ]. Some people have still tried to take advantage of others to get research results. One example is the Tuskegee study, a 40-year-long study in which hundreds of patients with a disease called syphilis were left untreated even though treatment was available [ 5 ]. Once news of this study reached the public, the United States released the Belmont Report in 1976. This made the rules for research in the United States today and has three main principles [ 4 , 5 ]. The first principle is respect, which means that all researchers must take the person’s feelings and choices into consideration when they interact with the person or their tissues. The second is beneficence, which means that the person must benefit from the study in a way that improves their health or life. The last principle is justice, which means that each person must be treated fairly, no matter who they are or what they look like. These rules must be followed by every researcher in the United States.
Henrietta Lacks and her Immortal Cells
In 1951, Henrietta Lacks was a young African American woman who lived in Baltimore, Maryland, and who had cervical cancer. She was treated at the Johns Hopkins Hospital in Baltimore. During her treatment, cancer cells were taken from her without her informed consent or knowledge. The doctors and scientists who used her cells never asked Henrietta if she wanted to donate cells. These cells (known as HeLa cells, for Henrietta’s name) were transformed in a lab into an immortal cell line [ 6 ]. An immortal cell line can multiply and continue to live outside the human body for long periods of time. This was an incredible discovery and very important for the future of human tissue research. Today, immortal cell lines are relatively easy to use, low cost, and allow researchers an unlimited supply of cells when grown correctly.
It is important to note that all human cells contain identifiable genetic information, and these cells will carry this information as long as they are kept alive by cell cultures. Henrietta’s immortal cell line therefore contains information that is specific to her, and that information is similar to that of her relatives. Today, researchers must get permission to collect genetic information and must keep this information private. As an African American woman, Henrietta was from a historically excluded group, especially in healthcare. She was not given credit for her cells until long after many scientific discoveries were made using them, and she and her family never received compensation for the unethical use of her cells [ 7 ]. She passed away from cancer without recognition. Her family discovered her contribution many years later, and they have been working to help celebrate her legacy in science and medicine. This is now a widely recognized example of injustice in science, and it is extremely important for scientists to remember, as they continue to use HeLa cells in research today.
The Legacy of Henrietta Lacks
Due to Henrietta Lacks’ contribution to modern medicine, millions of lives have been saved. Many vaccines, such as those for polio and COVID-19, were created using her cells [ 6 ]. Treatments for deadly diseases like HIV/AIDS, sickle cell anemia, and many types of cancer were discovered by scientists using HeLa cells [ 7 ]. This is why Henrietta Lacks is referred to as the mother of modern medicine. However, as we mentioned, she has not always been given credit for her contributions to science. Although it was legal at the time, it was unethical for her doctor to use her cells in this way without her informed consent [ 7 ]. Henrietta’s story is important because scientists of the future must know the wrongs committed by previous generations. This knowledge helps us learn from past mistakes and contributes to ethical scientific research that exhibits racial equity . Today, the Belmont Report protects humans in research [ 4 , 5 ]. Informed consent ensures respect for people. Justice ensures that the benefits and risks of research are distributed equally, without prejudice toward members of a particular gender or race. It is because of Henrietta Lacks that people of all ethnicities can have access to life-saving treatments and benefit from new scientific discoveries.
Informed Consent : ↑ The act of agreeing to something, or giving permission, after fully understanding what it involves.
Coercion : ↑ Persuading someone to do something, usually with threats or force.
In Vitro : ↑ Performed in a lab, outside of a living organism.
Ethical : ↑ Following a set of moral principles or guidelines that avoid harm and demonstrate respect for others as dictated by society.
De-Identified : ↑ A type of data where any information that can be used to identify a person or their relatives has been removed or replaced.
Institutional Review Board : ↑ A group formed to oversee biomedical research involving humans and ensure investigators follow ethical rules and guidelines.
Immortal Cell Line : ↑ Cells taken from a multicellular organism that can reproduce outside the body.
Racial Equity : ↑ The just, unbiased, and fair treatment of people of all races ( https://www.aecf.org/blog/racial-justice-definitions ).
Acknowledgments
JB was supported by the National Institute of General Medical Sciences of the National Institutes of Health [award T32GM086330]. KJ was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R35GM143044]. Research reported here is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
[1] ↑ Katt, M. E., Placone, A. L., Wong, A. D., Xu, Z. S., Searson, P. C. 2016. In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front Bioeng Biotechnol. 4:12. doi: 10.3389/fbioe.2016.00012
[2] ↑ Grizzle, W. E. 2019. Issues in the use of human tissues to support precision medicine. J. Health Care Poor. Underser. 30:66–78. doi: 10.1353/hpu.2019.0117
[3] ↑ Petrini, C. 2012. Ethical and legal considerations regarding the ownership and commercial use of human biological materials and their derivatives. J. Blood Med. 3:87–96. doi: 10.2147/JBM.S36134
[4] ↑ U. S. Department of Health and Human Services. Office of Research Integrity Introduction to Responsible Conduct of Research: Chapter 3. The Protection of Human Subjects. Available online at: https://ori.hhs.gov/content/chapter-3-The-Protection-of-Human-Subjects-IRBs (accessed June 2, 2023).
[5] ↑ Adashi, E. Y., Walters, L. B., Menikoff, J. A. 2018. The Belmont report at 40: reckoning with time. Am. J. Public Health. 108:1345–1348. doi: 10.2105/AJPH.2018.304580
[6] ↑ Adey, A., Burton, J. N., Kitzman, J. O., Hiatt, J. B., Lewis, A. P., Martin, B. K., Qiu, R., et al. 2013. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature 500:207–211. doi: 10.1038/nature12064
[7] ↑ Wolinetz, C. D., Collins, F. S. 2020. Recognition of research participants’ need for autonomy: remembering the legacy of Henrietta Lacks. JAMA 324:1027–1028. doi: 10.1001/jama.2020.15936
The use of human tissue in drug discovery
Affiliation.
- 1 AstraZeneca R&D Charnwood, Bioscience Department, Bakewell Road, Loughborough, Leicestershire, UK. [email protected]
- PMID: 20824358
- DOI: 10.1007/s10561-010-9201-9
Experiments conducted on human tissue samples are a key component of modern drug discovery programs and complement the use of animal tissue based assays in this process. Such studies can (i) enhance our understanding of disease pathophysiology, (ii) increase (or decrease) confidence that modulating the function of particular molecular targets will have therapeutic benefit (iii) allow comparison of the activities of different agents on particular mechanisms/processes and (iv) provide information on the potential safety risks associated with targets. All of this information is critical in identifying the targets that are most likely to deliver efficacious and safe medicines to address unmet clinical needs. With the introduction of new technologies, human tissue samples are also increasingly being incorporated into drug project screening cascades, including their use in high throughput assays. Improved access to human tissue would undoubtedly further extend the utility of this valuable resource in the drug discovery process.
- Drug Discovery / methods*
- High-Throughput Screening Assays
- Organ Specificity*

IMAGES
COMMENTS
Almost all the advances in medical care are based on research with human tissues. The availability of human tissues for research has led to many seminal discoveries in science and major developments in medical care as we describe in detail subsequently. Human tissues have been critical to the diagnostic characterization and subtyping of most ...
Mar 14, 2022 · Human tissue research can help by looking at the effects of drugs in donated bits of tissue (e.g. skin, cancer biopsies, blood) to check possible side effects before testing in human beings. Hundreds of researchers across the globe are using human tissue research to improve the way drugs are tested and developed. What is ex vivo research?
Human tissue is typically obtained post-mortem from people who are organ donors, as surgery remnants, and via blood or other bodily fluids. But accessibility is often cited as one of the main barriers of using human tissues and cells for scientific advancement. We’ve put together a resource for scientists who need more information about ...
Tissue for Research works with biomedical companies and academic institutions to provide annotated human specimens for diagnostic, drug, or medical device research or for basic research. Tissue for Research has an inventory of biospecimens or can collect samples from most diagnoses to meet a researcher’s specifications.
The Alder Hey organs scandal in the UK, involved the unauthorized removal, retention, and disposal of human tissue, including children's organs, from 1988 to 1995, and led to the Human Tissue Act 2004, and the creation of the Human Tissue Authority. 38,39 A number of cases in the US, have also highlighted important ethical issues related to the ...
Jan 31, 2024 · Informed consent is the process of obtaining permission from human participants to use their cells and tissues or otherwise include them in research studies. With informed consent, scientists can use human cells or tissues in experiments to learn more about the human body and to test new medicines. This article describes how these tissues are obtained, and the ethical concerns regarding the ...
Jul 8, 2022 · Many sources of human tissues exist, but researchers — particularly those making the transition from animal to human systems — may not be aware of how best to find quality sources of human tissues or how best to use them in their research. In this article, we discuss the advantages of using human tissues in research.
Research with human tissue offers the possibility not only of improving preclinical pharmaceutical research and safety assessment, but also of the substitution of some animal experiments. Surgically removed human tissue is discarded after pathological evaluation. This tissue would be of enormous val …
Apr 1, 2011 · David S. Wendler (patient rights): The tissues are not merely “a collective good.” The tissues are obtained from specific individuals, and their use involves the interests of the source individuals in at least 4 ways: (1) Obtaining samples can pose risks; (2) future research use of samples can expose individuals, and the groups to which they belong, to risks; (3) use of samples involves ...
Experiments conducted on human tissue samples are a key component of modern drug discovery programs and complement the use of animal tissue based assays in this process. Such studies can (i) enhance our understanding of disease pathophysiology, (ii) increase (or decrease) confidence that modulating …