Get Your ALL ACCESS Shop Pass here →


Candy Corn Experiment For Fall Science
Our dissolving candy corn experiment is a neat science experiment that is easy to set up with only simple supplies needed! Grab the free journal page and learn the simple science behind dissolving candy.

Candy Corn Experiment
Apply the scientific method , by investigating which liquid candy corn will dissolve fastest in. The dependent variable is the time it takes to dissolve the candy. The independent variable is the type of liquid. Learn more about variables in science.
Supplies:
- Candy Corn (look for the gumdrop-like pumpkins too!)
- Peeps (ghosts and pumpkins)
- Various liquids – water, vinegar, oil, seltzer, lemon juice, soda
- Science journal page (below)
💡 TIP: I used my iPhone as a timer for the dissolving candy experiment but any timer will do. You could also make a water clock timer for a fun extension activity!
Instructions:
STEP 1. Measure and fill clear cups with each of the liquids you are using. We used 5 liquids: cold water, hot water, oil, vinegar, and seltzer as our potential solvents.
STEP 2. Place the candy in each of the cups and start the timer. Observe what happens to the candy in each liquid.

Dissolving Candy Corn Results
We did two rounds of experiments. In the first round, we used Peep candy (both pumpkins and ghosts). In the second round, we used candy corn.
Using two different types of candy was perfect because we quickly discovered that Peeps float, while candy corn sinks. They also have significantly different dissolving times, which raises some interesting questions.
It was particularly fascinating to observe how the waxy layer on the surface of the candy corn separated from the candy first. We repeated this part of the experiment a few times because my son found it so intriguing!
Which liquid dissolves candy corn the fastest? Make your predictions and test your theories! This is a much quicker dissolving candy experiment if you need immediate results.

Free Printable Science Journal Page

The Science Of Dissolving Candy Corn
Candy corn dissolves in water because water effectively breaks apart the sugar molecules in the candy. Both water and sugar are polar molecules. When candy corn is added to water, the water molecules surround the sugar molecules and pull them away from each other, causing the candy to dissolve.
In warm water, the higher temperature increases the movement of water molecules. This energetic movement allows the water molecules to interact with the sugar molecules in the candy corn more quickly, resulting in faster dissolving.
In cold water, the lower temperature means the water molecules move more slowly, leading to slower interactions with the sugar molecules and thus a slower dissolving process.
Oil, unlike water, is a non-polar substance. No significant attraction exists between the non-polar oil molecules and the polar sugar molecules in candy corn. As a result, oil cannot break apart the sugar crystals, and candy corn does not dissolve in oil.
Polar substances, like the sugar in candy corn, dissolve in polar solvents like water, but not in nonpolar solvents like oil.
Vinegar is a polar substance containing water and acetic acid, enhancing its polar nature. Is vinegar a better solvent than water for dissolving candy corn? Let’s find out!
What Is The Scientific Method For Kids?
The scientific method is a process or method of research. A problem is identified, information about the problem is gathered, a hypothesis or question is formulated from the data, and the hypothesis is tested with an experiment to prove or disprove its validity.
Sounds heavy… What in the world does that mean?!? It means you don’t need to try and solve the world’s biggest science questions! The scientific method is all about studying and learning things right around you.
As children develop practices that involve creating, gathering data, evaluating, analyzing, and communicating, they can apply these critical thinking skills to any situation.
READ MORE HERE: Using The Scientific Method with Kids
Note: The use of the best Science and Engineering Practices is also relevant to the topic of using the scientific method. Read more here and see if it fits your science planning needs.
Helpful Science Resources To Get You Started
Here are a few resources to help you introduce science more effectively to your kiddos or students and feel confident when presenting materials. You’ll find helpful free printables throughout.
- Best Science Practices (as it relates to the scientific method)
- Science Vocabulary
- 8 Science Books for Kids
- All About Scientists
- Science Supplies List
- Science Tools for Kids
- Join us in the Club
Turn It Into A Science Project
Science projects are an excellent tool for older kiddos to show what they know about science! Plus, they can be used in various environments, including classrooms, homeschools, and groups.
Kids can take everything they have learned about using the scientific method , stating a hypothesis, choosing variables , making observations, and analyzing and presenting data.
Want to turn this experiment into an excellent science fair project? Check out these helpful resources.
- Science Project Tips From A Teacher
- Science Fair Board Ideas
- Easy Science Fair Projects
Bonus Candy Corn Activities
Candy corn oobleck.
Make a batch of our oobleck recipe and read about the science behind it. Add a handful of candy corn and observe the cool science behind the activity and the dissolving candy! Makes great tactile sensory play.
Candy Corn Slime
Our soft and squishy candy corn fluffy slime is perfect for fall slime making activities with kids. The base for this candy corn slime uses glue, shaving cream, baking soda, and saline solution.

More Fun Candy Experiments To Try
- Floating M&M
- Pumpkin Skittles Experiment
- Starburst Candy Slime
- Dissolving Candy Fish
- Gummy Bear Osmosis

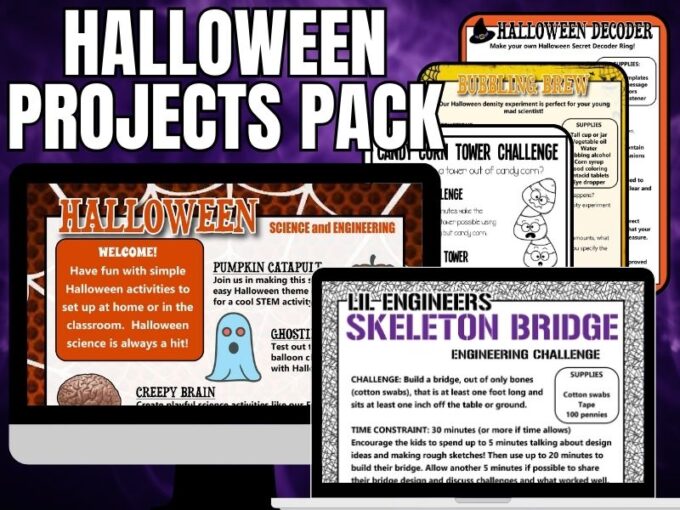
Printable Halloween STEM Activities Pack
150+ Pages of Halloween-themed materials !
This Halloween project pack is suitable for home, school, and group use for kids in grades Kindergarten through elementary but is scaleable for many ages and abilities.
What’s Included:
- 25+ Halloween theme science and STEM activities with printable sheets, instructions, and useful information all using easy-to-source materials perfect for limited-time needs. Includes a Halloween engineering pack with fun, problem-based challenges for kids to solve!
- The skeleton activity pack includes building a skeleton challenge and a coding challenge! Try a skeleton bones bridge-building STEM challenge!
- Halloween theme brick printable activities for hands-on learning with math that is perfect for early finishers or home fun and reinforces basic math concepts.
- Bonus fun pack includes games and activities to round out your Halloween theme activities such as I Spy, bingo, matching, Would You Rather cards, scavenger hunt, word search, A-Z Halloween hunt, and a coloring page.
- Halloween STEAM Pack includes artist-inspired projects by Warhol, Lichtenstein, and more!
- Pingback: Halloween Engineering Projects for Kids - Left Brain Craft Brain
- Pingback: The Best Halloween STEM for Preschool and Kindergarten - Natural Beach Living
- Pingback: Magical Dancing Corn Thanksgiving Science Experiment
Can’t wait until I have a grandkid to explore this with.
- Pingback: 30+ Halloween Games & Activities for Kids: Great for Classroom Halloween Parties!
Comments are closed.

Subscribe to receive a free 5-Day STEM Challenge Guide
~ projects to try now ~.


Candy Corn Experiment – 2 Age Levels
Sharing is caring!

Our candy corn experiment is divided into two age groups – preschool through elementary and middle through high school. Worksheets are included for each level; please feel free to use them as they best fit your child. For the younger scientists, answers can be drawn, written, or dictated.
A candy corn experiment has multiple chemistry lessons for all ages, the amount of detail we go into about that chemistry is what distinguishes the two levels.
Older students will test to see if there are differences in temperature and pH levels. Below is a photo of our final results of our candy corn experiment.

Table of Contents
Before starting the experiment, you might want to read this article about candy corn from the College of Agricultural & Environmental Studies at the University of Georgia Cooperative Extension. (Older students can read it on their own.)
Also, this candy corn experiment is an ideal activity for giving our children extra time to take the experiment further with their own ideas. Allow extra time for your child to test other liquids. Our children become comfortable with science by trying their ideas and making new observations.
Candy Corn Experiment – Younger Level
Please take a look at the vocabulary words with your children before starting.
Explain what they will be doing in their science experiment. Tell them they will watch to see if the candy corn dissolves in each liquid.
They will see some chemical reactions, just like a real chemist!
As you complete the activity or discuss what happened, incorporate the words into your discussion. Encourage your children to say the words. In the free printable that accompanies this activity, are two vocabulary worksheets. If your child struggles to hold a pencil and write the words, here are some alternate ideas:
- Skip the writing!
- Have your child trace the letters with their fingers.
- Use dyed salt trays to write the letters. See this site for a how-to .
- Form the letters out of playdough.
- Use magnetic letters to write the words
Vocabulary for Younger Ages
The word dissolve means to put something solid into something wet, and the solid breaks apart or disappears. When we cook soup and add salt to the hot soup, the salt breaks apart. We cannot see it, but we can taste it. The salt dissolved into the soup.
A chemical reaction is when we mix two things and something new is made. When we bake a cake, we mix flour, sugar, eggs, and other ingredients. Then, we put it in the oven, and the heat helps turn those ingredients into a cake!
Before Getting Started with the Candy Corn Experiment
Before getting started with this candy corn experiment, here are a few tips:
- Download the printable pack and gather the items below
- You will need to help keep track of the start time and how much time passes before your child starts to see changes. It doesn’t need to be super-precise, but the idea is to be able to talk about which liquid took the longest to begin dissolving the candy and which worked the fastest. This helps children about the types of observations scientists make.
- In the results box on the worksheet, ask your child to draw what they see in each cup. Then, write the number of minutes it took to dissolve.
- If you have a magnifying glass available, your child can use it to take a closer look at what is happening in each cup.
Ask a few questions before starting:
- Review the vocabulary pages for the words observe, dissolve, and chemical reaction.
- Smell each liquid. How does it smell?
- Taste a very small amount of each. How does it taste? Is it sour or sweet?
- What do you think will happen when you put the candy in the water? Lemon juice? Vinegar? Soda? For older students, fill out the My Predictions sheet.
As you are setting up the experiment, ask your child to:
- Count out the pieces of candy. (Use the same type and number of candy in each cup.)
- Help them measure out the lemon juice, vinegar, and soda. A grown up should handle the hot water.
This packet contains the printables for preschool-early elementary students. The candy corn experiment is included in this resource. The printable for older students is at the end of the experiments below.
Your email address will be added to our email community. You may unsubscribe at any time by clicking the unsubscribe button in our emails.
Candy Corn Experiment – Younger Version
- Thermometer
- Magnifying glass Optional
- Candy corn – 1 piece per liquid tested
- Clear plastic cups or drinking glasses, label each so you know what liquid is in each. You will need 1 tall glass for the vinegar and baking soda mixture.
- 1/3 cup lemon juice
- 1/3 cup vinegar
- 1/3 cup 1/3 cup hot water (a grown up must handle this)1/3 cup of clear soda pop (We used clear so we could more easily see the result.1/3 cup of orange juicecrayons, colored pencilsworksheetspencilphone camera to take video and/or photosMagnifying glass (optional)
- 1 teaspoon baking soda
- 1/3 cup Hot water (This should be handled by adults only. We used boiling water and let it cool for about 1 minute.)
- 1/3 cup clear soda (pop)
- 1/3 cup orange juice
- crayons or colored pencils
- phone camera or video
- magnifying glass (optional)
Instructions

- Help your child pour each liquid into a clear plastic cup or glass.

- Record the start time and have your child drop one piece of candy in each cup. Now, the fun observations begin!
- Add one teaspoon of baking soda to the vinegar in the tall glass.
- If you have a magnifying glass, help them use it to examine the chemical reactions closely.

- After 5 minutes, ask your child to draw their observations in each cup using their Lab Observation Sheet.
- If age-appropriate, have your child answer the questions on the My Observations page.
What is Happening in the Candy Corn Experiment
Here is some information to explain to your child what is happening in the candy corn experiment. Use this information, if it is appropriate for your child’s comprehension level.
Pictured here is candy corn in soda. The white material floating on the top is the confectioner’s glaze on the outside of the candy corn.

Candy corn is mostly made of sugar, corn syrup, and binders. Soda pop is carbonated, meaning it contains dissolved carbon dioxide gas under pressure. When you add candy corn to soda, some of the carbon dioxide will come out of solution in the form of bubbles. This can lead to fizzing and the release of gas, creating the bubbles we see.
When you place candy corn in vinegar, the sugar and corn syrup in the candy corn begin to dissolve in the water component of the vinegar.
The bubbles in the baking soda and vinegar mixture are from the reaction between the sodium bicarbonate in the baking soda and the acetic acid in the vinegar.
Candy Corn Experiment – Middle & High School Edition
Dependent and independent variables in the candy corn experiment.
Before doing this experiment, check out the history of candy corn !
If you are looking for a way to add a seasonal touch to a chemistry experiment for your older students, check out this activity or this one involving pumpkin candies !
In this candy corn experiment, we will investigate if the pH level and temperature of a solution changes when a salute is added to the solvent. In this experiment, the candy corn in the solute and the solvents are vinegar, 91% isopropyl alcohol, hydrogen peroxide (H202), vinegar & baking soda, lemon juice, orange juice, and Starry soda. (You may opt to use other solvents or a subset of the ones we used in this experiment.)
We will test the before and after pH to see if adding the sugar of the candy corn changes the pH level of the final solution.
Does the sugar and other ingredients in the candy corn affect the pH levels?
Will there be any dramatic temperature changes due to an endothermic or exothermic reaction?
We will also measure the starting temperature of each solvent and the temperature of the solution once the solute (candy corn) is added.
In this experiment, the independent variable is liquids we choose to use (vinegar, hydrogen peroxide, etc.) We have several dependent variables we can measure:
- Rate of Dissolution : You might measure the time it takes for the candy corn to completely dissolve in each liquid. This would involve recording the time it takes for the candy corn to disappear or become fully dissolved.
- Change in Mass : You could weigh the candy corn before and after it’s dissolved in each liquid to determine if there is a change in mass.
- Change in Appearance : You might observe and describe any changes in the candy corn’s appearance, such as color alterations or texture changes, as it dissolves in different liquids.
- pH Level : If you’re using liquids with varying levels of acidity, you could measure the pH level of each liquid before and after dissolving the candy corn to see if there’s a change in acidity.
- Bubbling/Fizzing : If some of the liquids cause fizzing or bubbling due to a chemical reaction, you could measure the intensity of the fizzing as it relates to the dissolution process.
We opted to measure temperature, pH level, and time to completely dissolve.
Precautions and Note to Parents and Teachers
We will be using hydrogen peroxide, Isopropyl alcohol, and shower cleaner. You may want to use a Dawn dishwashing liquid and forgo using the shower cleaner, or you may want to try another household cleaner you use. The use of the household cleaners is optional.
Please use gloves and safety glasses . Keep all substances away from heat.
Also, you may want to add hot water (just as it starts to boil) to this experiment.
Candy Corn Experiment – Measuring pH level, temperature, and dissolution rate
- 80 ml vinegar or 1/3 cup
- Candy corn or 1/3 cup
- 40 ml vinegar (for use in a tall glass) We will be adding baking soda to this, so we lessened the amount of vinegar.
- 80 ml hydrogen peroxide or 1/3 cup
- 80 ml Isopropyl alcohol (We used 91%. Either is OK.) or 1/3 cup
- 80 ml orange juice or 1/3 cup
- 80 ml fresh squeezed lemon juice or 1/3 cup
- 80 ml shower cleaner (We used Zed.) or 1/3 cup
- Glass beakers or plastic cups and at least one tall glass container for mixing the baking soda and vinegar.
- Paper for labeling each liquid
- pH paper (20 pieces per set up) and the pH chart that should have been included with the paper
- Marker, pencil
- Lab sheets from our printable pack
- Paper towels to wipe off the thermometer
- Put on gloves and safety glasses.
- Gather all materials.
- Bring all liquids to room temperature before starting.
- Make labels for the vinegar, vinegar and baking soda, hydrogen peroxide, Isopropyl alcohol, lemon juice, soda, orange juice, shower cleaner.
- Measure out the remaining liquids.
- Arrange the containers and put their label in front.
- Next, dip one pH paper in each liquid, checking it against the pH chart.
- Place the pH paper on the table to dry. Place the "before" strip on the left side of the label. We will let it dry, then record it on the lab sheet.
- Take the temperature of each liquid and write it down on the lab sheet in the Before Temp area. Be sure to wipe the end of the thermometer after each reading before dipping it into the next liquid.
- Again, make sure your student(s) have on lab glasses and gloves.
- FIRST, add the candy corn to the vinegar setup where you will add the baking soda. Take the temperature of the vinegar after the candy corn only has been added. Make a note of this temperature. So, you'll have 3 readings for the vinegar-baking soda mix.
- Add 1 piece of candy corn to each liquid.
- Make sure the thermometer is in the glass with the vinegar. Read the temperature and watch the temperature as the baking soda is added to the vinegar.
- What is happening to the temperature of this mixture? Have someone record this temperature. Continue to watch the temperature for a few more seconds.
- One person should record what happened to the temperature.
- Next, start measuring the other liquids' temperature while observing any changes like bubbles or dissolution. Important: wipe off the thermometer after dipping in each glass.
- Dip a second pH strip into each solution and place on the right side of the labels.
- Continue filling out the data portion of the lab sheet.
- Complete the remainder of the questions and sections of the worksheets.
Photos of Our Candy Corn Experiment


Video of the Endothermic Reaction During the Candy Corn Experiment
What Was Observed
Two of the most notable outcomes of this experiment were the endothermic reaction in the baking soda-vinegar mixture indicated by the drop in temperature and the before and after pH values.
The biggest observable pH difference was in the vinegar and baking soda. This is because the vinegar alone is acidic, but when baking soda, an alkaline, is added, the mixture becomes more base.
The other mixtures did not have a significant change in their pH readings because sugar in this low amount did NOT affect the pH level in our experiment. Sugar is neither acidic or base; it has a pH of 7. (However, high amounts of sugar in our bodies does negatively affect our bodies. One effect is a lower amount of sodium and potassium in our bodies. This, in turn, can affect the body’s ability to absorb water. High amounts of sugar in our body will affect our body’s pH. So, eating large amounts of sugar is just not good for our bodies!)
The temperature changed most significantly in the vinegar and baking soda mixture.
We measured the temperature of the vinegar alone. Then, we measured the vinegar when the candy corn was added. The temperature did not change. Lastly, we measured the temperature once the baking soda was added. So, three temperature measurements were taken for the baking soda-vinegar mixture.
When baking soda (sodium bicarbonate, NaHCO3), which is more base (alkaline), mixes with vinegar, which contains acetic acid (CH3COOH) mix, an acid-base reaction occurs.
Specifically, the acetic acid in vinegar reacts with the sodium bicarbonate in baking soda to produce water (H2O), carbon dioxide gas (CO2), and sodium acetate (CH3COONa). The chemical equation for this reaction is:
CH3COOH + NaHCO3 -> CH3COONa + H2O + CO2
Chemical bonds break apart (vinegar and baking soda) and new bonds are made in the products of water, carbon dioxide, and sodium acetate. This breaking apart of bonds takes energy, which is taken from the surrounding area, resulting in a lower temperature.
This is called an endothermic reaction. You can read more and do another endothermic reaction in this post .
The fizzing is the production of carbon dioxide gas during the reaction.
Sodium acetate is typically left behind as a solid in the solution once the reaction is complete.
Some of our students were surprised that the candy corn dropped in vinegar didn’t dissolve quickly. They hypothesized that the vinegar would dissolve the candy corn quickly. But the results were different!
To understand this, let’s look more closely at the chemistry of vinegar. Vinegar is primarily made up of water with a small amount of acetic acid, usually between 3% and 5%. Its ability to dissolve things depends on whether those substances can mix well with water.
Vinegar is effective at dissolving substances that are water-soluble, such as low-molecular-weight sugars and certain types of salts. This dissolution process is aided by vinegar being an acidic solution, meaning it has a low pH. The acidity helps break down and dissolve specific salts that would typically remain insoluble in plain water.
Vinegar itself is hydrophilic, which means it mixes well with water. However, vinegar doesn’t dissolve hydrophobic compounds as well. Hydrophobic means these substances avoid water. This includes things like colorful dyes, oil, and large, complex molecules like plastics.
Vinegar does not react well with sugar. As we observed in this experiment, the candy corn dissolved more slowly than it did in some of the other liquids. This is because the candy’s composition doesn’t easily interact with the acetic acid in vinegar, resulting in a slower dissolution rate.
The printable below is for older students.
I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.
- Skip to secondary menu
- Skip to main content
- Skip to primary sidebar
- Skip to footer
Tina's Dynamic Homeschool Plus
dy•nam•ic constant change, progress, activity
Fun Candy Corn Stem Activity Which Liquid Dissolves Candy Corn Faster
October 18, 2023 | Leave a Comment This post may contain affiliate links. For more information, please see my full disclosure policy .
Share This!
The candy corn stem activity I have for you below, whether you are a team candy corn lover or team hater still makes for a fun science experiment. Also, you’ll love my pages 5 Fun Candy Science Experiments and Unit Study & Homemade Lollipops and Fall Season Unit Study {Pumpkins, Leaves, Corn, & More}.
When you are learning about fun fall topics like flint corn which was so important to the Native Americans look for fun hands-on activities.
This activity is a great introduction to science concepts like making hypotheses, observing, setting up variables, and recording data.
Giving even more value to what a history lesson would just be.

I will give you a little bit more detail about the science behind why certain liquids work so much faster at dissolving the sugar than others at the bottom of the post, after the activity instructions.
Indian Corn was one of one of three types of corn cultivated by Native Americans in the northern part of the US as a staple.
It is one of the oldest types of corn and comes in a variety of colors.
Ears can be single colors of white, red, blue, gold, yellow, or black, but most are beautiful mixes.
Candy corn comes in fun traditional colors of yellow, orange, and white representing the colors of the fall harvest.
It is a fun representation of a corn kernel and so appropriate to go along with this study.
Table of Contents
5 Indian Corn Facts
First, look at these facts.
- “Indian corn” isn’t exclusive to the North American continent. Experts believe it also grew in China, India, and South America for centuries.
- The earliest Native Americans to cultivate corn were the Pueblo people of the American Southwest, later corn became a staple for many tribes like- Creek, Cherokee, and Iroquois.
- Indian corn can be ground to make flour (or cornmeal) or the whole kernel can be used for popcorn.
- Corn is one of “’The Three Sisters” crops, which are planted together in a shared space. They are maize, beans, and squash and are planted because they benefit one another and Native Americans believe that they nurture each other like a real family when planted together.
- Flint corn has a very low water content, making it more resistant to freezing than other vegetables. As a matter of fact, it was the only crop in Vermont to survive the winter of 1816, known as “the year without a summer.”

Also, look at some of these books about corn.
6 Books About Corn and Resources for Multiple Ages
Books for kids who want to learn about corn.

Corn Is Maize: The Gift of the Indians
With simple prose and beautiful illustrations, award-winning author-illustrator Aliki tells the story of how Native American farmers thousands of years ago found and nourished a wild grass plant and made corn an important part of their lives. They learned the best ways to grow and store and use its fat yellow kernels. And then they shared this knowledge with the new settlers of America.

Find out everything about this versatile and important grain—its history as a crop, the four main types, and how we grow and use it to make everything from food to paper to medicine!

From Kernel to Corn (Start to Finish, Second Series)
How does a corn seed become corn on the cob? Follow each step in nature's cycle―from planting to picking and eating―in this fascinating book!

Glass Gem Cherokee Indian Corn, Flint Corn 100 Seeds
GROW. For the best results, it's essential to cover your seeds with about one inch of soil and tamp down firmly before watering lightly.You can plant your seeds in either rows or hills. If you are planting them in rows, plant one seed every 4 inches in rows that are 18 to 24 inches apart.

Farm Anatomy: The Curious Parts and Pieces of Country Life
Learn the difference between a farrow and a barrow, and what distinguishes a weanling from a yearling. Country and city mice alike will delight in Julia Rothman’s charming illustrated guide to the curious parts and pieces of rural living. Dissecting everything from the shapes of squash varieties to how a barn is constructed and what makes up a beehive to crop rotation patterns, Rothman gives a richly entertaining tour of the quirky details of country life.

The Story of Corn: It Starts with a Seed
How does a seed become a yummy ear of corn? A farmer plants seeds. The seeds change into plants. Plants grow. Soon cobs grow on the plants. Learn about the life cycle of corn step by step.
Also, look at some of these hands-on activities.
Indian Corn Hands-on Activities
- I love how bubble wrap gives a nice corn texture to this Corn Craft – Preschool .
- Geronimo Stilton Field Trip to Niagara Falls Summary And Fun Corn Craft
- Paper bag crafts are a super inexpensive craft base and these Stuffed Paper Bag Indian Corn are just adorable.
- Popcorn Science Mini Unit Study Which Brand Pops the Best.
- Fun Corn Life Cycle Preschool Sensory Bin and Printable Lifecycle Foldout.
- Gather up pinecones on your next nature walk and create a Pinecone Indian Corn Craft .
- Have you ever thought to use a whole ear of corn as a rolling pin? Check out these Harvest Sensory Ideas .
In Farm Anatomy I was able to find a section on corn, aren’t these illustrations beautiful?

Finally, look at this fun candy corn stem activity.
Fun Candy Corn Stem Activity
While this activity is simple it gives your child a chance to exercise their science muscles by learning about some basic concepts through making guesses on the outcome (a hypothesis) as well as observing and recording their findings.
You will need:
- Various liquids
- clear glasses or bowls
- Timer/stopwatch

First, decide what liquids you want to use for this activity, I recommend choosing 4-6 different types.
It can be warm or cold water, salt water, vinegar, rubbing alcohol, oil, and clear soda.
Place two or three candy corn in each container.

Add enough of each liquid to cover the candy corn completely.
Heat your water up in the microwave for 30 seconds or just use hot water from the tap.

Make a label for each liquid you use and place it in front of the proper containers so they don’t get mixed up.

Observe the candy corn and note what each one is doing, set a stopwatch timer and see how long it takes for the first liquid to dissolve or time each.
We noticed changes begin right away in the very warm/ hot water, it immediately.

The vinegar was just a little behind the warm water.

And the oil and the rubbing alcohol seemed to do nothing at all.
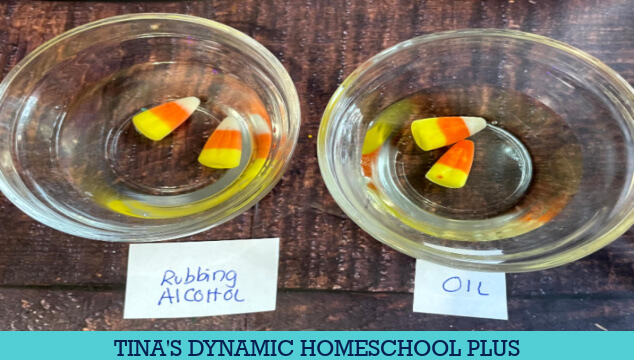
The very warm water was the clear winner.
It took about 15 minutes to become almost completely dissolved.
Now the science behind this is that water and vinegar are both polar molecules, and so is sugar.
So, when you add those liquids to the sugary candy corn it pulls away at it breaking it down quickly.
The heat makes these molecules move around even faster.

Reader Interactions
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *

This site uses Akismet to reduce spam. Learn how your comment data is processed .

Privacy Policy | About Me | Reviews | Contact | Advertise
Tina Robertson is a participant in the Amazon Services LLC Associates Program, an affiliate advertising program designed to provide a means for sites to earn advertising fees by advertising and linking to amazon.com . As an Amazon Associate, I earn from qualifying purchases.

- Pre-K Printable Fun
- Become a Member

Candy Corn Jar Science Experiments
Candy corns are a divisive topic during Halloween season. Some people love them. Others? Not so much. There's one thing we can all agree about regarding this pyramid-shaped, waxy-textured, multi-colored confection—they're perfect for science experiments!
All you need is a few materials and you're ready to make predictions, record observations, and share the results of this series of candy corn experiments inspired by Coffee Cups and Crayons !
Note from Early Childhood Educator Elyse Handel:
"Make predictions prior the experiments! Have the children talk about what they know about water, heat, etc. See if they can make guesses as to what might happen to the candy corn based on previously experiences using a microwave, water, and freezer."

- Bag of candy corn
- 3 or more jars (or drinking glasses)
- Paper
- Writing utensil
Will candy corns dissolve in water?
- Use your writing utensil and paper to record what you think will happen when candy corns are added to a jar of water.
- Add water to your jar.
- Drop some candy corn into the jar.
- Record what you see.
- Repeat the experiment with different temperatures of water. Did anything change?
What happens to candy corns in the cold?
- Write (or draw) your prediction.
- Add five or six candy corns to an empty jar.
- Place in the freezer for 20 minutes.
- Remove the jar from the freezer.
- Record your observations. Is the color the same? Does it sound the same when you shake it? What else is different?
- Repeat the experiment and adjust the amount of time the candy corns are in the freezer. Did anything change?
What does heat do to candy corn?
- Heat in the microwave for 45 seconds.
- Be careful! The jar and its contents could be hot! Have a grown-up use a pot holder to remove the jar.
- Record your observations. What do the candy corns look like? How long did it take for it to change? Does it smell different?
- Repeat the experiment with different power level settings.

You can see all of our at-home activities on the blog or on Pinterest .
- Buy Tickets
- Privacy Policy
- Internships
- Accessibility
Official Partners
- Plan Your Visit

3000 North Meridian Street Indianapolis, IN 46208-4716
317-334-4000
Sign Up for Email
By signing up I agree that I am 13 years or older, or I have my parent or guardian's consent.

Candy Science Experiments & STEM Challenge

These science experiments are the perfect way to get rid of leftover Halloween candy or to just add something sweet to your science curriculum!
Skittles Density Rainbow
In this fun science experiment you use Skittles candy to create a rainbow in a clear glass or jar. It’s perfect to do after Halloween with leftover candy or in the spring around St. Patrick’s Day. It is a simple way to teach students about density.
Materials Needed:
Skittles candy 6 small glasses or jars eye dropper hot water a tablespoon
Place 2 Tablespoons of hot water in each of 5 glasses.
Place the following number of Skittles in each of the 5 glasses:
2 red 4 orange 6 yellow 8 green 10 purple

Wait for the Skittles to dissolve. If you need to speed up the process, microwave each cup up to 30 seconds.
While the Skittles are dissolving, I have students record how many Skittles of each color we are using for the experiment.
We then discuss which color they think has the most sugar and would therefore be the most dense. I then have students predict what color they feel should be on the bottom of the rainbow (the purple because it is the most dense and therefore the heaviest).

Once the candy is dissolved, allow the water to cool (cold water is more dense than warm water).
Have students help you arrange the glasses from most dense to least dense.
Using the eye dropper, transfer the purple water to a new glass or jar.
Then add the green water to the new jar using the eye dropper and SLOWLY dribble the water along the inside of the glass. If you dump the water in or add it too quickly they will mix together and the rainbow will not form.

Continue to slowly add the remaining colors in order using the eye dropper to form the Skittles density rainbow.

After the experiment, I have students draw pictures of the rainbow and write what they learned (The water with the most Skittles was the most dense because it had the most sugar. The water layered from most dense to least dense.)

Candy Sink or Float Experiment
This simple and fun candy sink or float experiment is the perfect way to teach students about the scientific method and get rid of leftover Halloween candy.
a variety of candy bars or candy bowl of water recording page
Show students the candy and pose the question “Do you think the candy will sink or float?”.

Allow students to pick up and observe the candy bars before making their predictions (hypotheses) about each one.
I had each student make their own prediction and record it on a page. They drew a picture beside each candy to show their prediction (drawing a picture and knowing where to place it in the bowl helps reinforce the concept of sink or float).

Place each candy bar in the bowl of water to test whether it sinks or floats.
Here were the results from our test:
Reese’s Peanut Butter Cup – sank

Three Musketeers Bar – floated

Tootsie Roll – sank

Kit Kat Bar – floated

York Peppermint Patty – sank

100 Grand – sank

As we tested each candy bar students recorded the results of the experiment on their pages.

After all the results were recorded it was time to analyze the results. We broke open the candy bars to observe the insides and figure out why the Kit Kat bar and Three Musketeers bar floated. We observed that they both had more air pockets inside and were therefore less dense than water which made them float.
What Dissolves Candy Corn Experiment
Candy corn is one of my favorite treats so I always have a lot of it on hand at Halloween which is why I do several candy experiments with it. Unfortunately it is ALL sugar and one of the worst candies for you, especially for your teeth :(. This experiment is a fun way for students to learn what dissolves candy corn (sugar) the quickest.
Please note that the liquids used for testing can be modified (warm water works best so I advise that be used). These were the ones we had on hand and the students wanted to test.
candy corn glass jars or glasses room temperature water vinegar oil pop (or some of you may call it soda, we used Sprite because it was clear)
Fill each jar or class with the same amount of each liquid. We used 1/2 cup which was more than enough. Label each.
Ask students to predict which liquid they think will dissolve candy corn the quickest.
We recorded our results before beginning the test (experiment).

Drop a piece of candy corn in each jar at the same time.

Observe the jars/glasses. You should observe that the candy corn begins to dissolve in the warm water fairly quickly followed by the vinegar. It was bubbling in the pop (soda) but not dissolving quite as quickly as it was in the water and vinegar. It didn’t seem to do much at all in the oil.
You can either wait to see which liquid completely dissolves the piece of candy corn first or take out the pieces after a while to more closely observe the results (this is what I did to make it easier for students to observe and record the results).

In our experiment the warm water dissolved the candy corn the quickest followed by the vinegar, pop, oil.
I had students record the results of the candy experiment on their pages.

Students can also record actual photos of the experiment using Pic Collage.

The Science Behind It:
Water molecules have powerful magnetic properties that break apart the bonds that hold sugar molecules together. They can actually insert themselves between the sugar molecules which is why the sugar (candy corn) breaks apart. Eventually they insert themselves in between all of the sugar molecules dissolving the candy corn.
What Dissolves Candy Corn Experiment (Hot or Cold Water)
Another version of the dissolving candy corn experiment to try is whether hot water or cold water dissolves candy corn the quickest. This is an easier version for younger students.
candy corn 2 clear glasses or jars hot water cold water
Before beginning the experiment have students hypothesize which water they think will dissolve the candy corn the quickest and record their predictions.

Place the same amount of hot and cold water in each jar or glass (we used 1/2 cup). Place a piece of candy corn in each glass at the same time.

The hot water starts to dissolve the candy corn piece right away.
Observe the glasses until a piece of the candy corn is dissolved.

Record and discuss the results. Students can draw the results or take photos with their iPads and record the results in an app such as Pic Collage.

The heat in the hot water makes the molecules move faster so the water molecules are able to break up the sugar (candy corn) molecules at a faster rate.
Candy Corn Stacking STEM Challenge
There is a legend that says candy corn got its name because when you stack it up just right it looks like an ear of corn. In this STEM challenge students see how high they can stack pieces of candy corn before the stack collapses.
candy corn (approximately 60-75 pieces per student)
Give each student a bag or pile of candy corn.
Explain that they are to stack the candy corn in a circle to resemble a piece of corn and that their base (bottom) must have at least 10 pieces of candy corn.

On your signal students begin to build their candy corn stacks.

Once their stack falls or pieces fall in to the center (they usually fall in on themselves) they have to stop and count how many pieces of candy corn they were able to stack.
You can give students a certain time period to stack their candy corn or allow them to stack until all stacks fall.

Have students record how many pieces of candy corn they were able to stack. You can also have them reflect on what was the easiest and most challenging parts of stacking the candy corn.
Discuss the findings. The winner is the student who was able to stack the most pieces of candy corn.

If you would like to use the candy science experiment printable pages from this post with your students CLICK HERE .
You may also like:.

Digital Candy Corn Number Sense Puzzles – No Prep!
Pumpkin science & stem ideas, apple science experiments & stem activities, water cycle / rain cycle experiments, snow science experiments, have engaging science experiments and stem activities throughout the entire school year with this money-saving science & stem bundle .

Pin it for later!

Share this:

Hi! Thanks for stopping by!
I’m Tina and I’ve taught preK and K for 20+ years. I share fun and creative ideas that spark your students’ love for learning.
-Featured Products-

Follow my TpT store to find out about new resources & freebies!
-Affiliate Disclosure-
Some of the Amazon links on Lessons for Little Ones are affiliate links. If you purchase a product after clicking an affiliate link, I receive a small percentage of the sale for referring you, at no extra cost to you. Purchasing through affiliate links is an easy, painless way to help out your favorite bloggers. Thank you so much for your continued support!
© 2023 Lessons for Little Ones by Tina O’Block
Privacy Policy | Designed by Megan Milton
© 2023 Lessons for Little Ones by Tina O’Block
Discover more from Lessons for Little Ones by Tina O'Block
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading

IMAGES
COMMENTS
Aug 16, 2024 · Candy Corn Experiment. Apply the scientific method, by investigating which liquid candy corn will dissolve fastest in. The dependent variable is the time it takes to dissolve the candy. The independent variable is the type of liquid. Learn more about variables in science. Supplies: Candy Corn (look for the gumdrop-like pumpkins too!)
Candy Corn Experiment – Younger Level. Vocabulary for Younger Ages; Before Getting Started with the Candy Corn Experiment. This packet contains the printables for preschool-early elementary students. The candy corn experiment is included in this resource. The printable for older students is at the end of the experiments below.
Oct 18, 2023 · The candy corn stem activity I have for you below, whether you are a team candy corn lover or team hater still makes for a fun science experiment. Also, you’ll love my pages 5 Fun Candy Science Experiments and Unit Study & Homemade Lollipops and Fall Season Unit Study {Pumpkins, Leaves, Corn, & More}.
Oct 17, 2024 · Preschoolers have a ton of questions and science experiments fosters thinking, exploration, and investigation. With autumn approaching it’s the perfect time to bring in holiday themed experiments. This candy corn experiment explores density and makes for a great addition to a Halloween theme.
"Make predictions prior the experiments! Have the children talk about what they know about water, heat, etc. See if they can make guesses as to what might happen to the candy corn based on previously experiences using a microwave, water, and freezer." Materials. Bag of candy corn; 3 or more jars (or drinking glasses) Water; Microwave; Freezer ...
There is a legend that says candy corn got its name because when you stack it up just right it looks like an ear of corn. In this STEM challenge students see how high they can stack pieces of candy corn before the stack collapses. Materials Needed: candy corn (approximately 60-75 pieces per student) Give each student a bag or pile of candy corn.