Practical Biology
A collection of experiments that demonstrate biological concepts and processes.


Observing earthworm locomotion

Practical Work for Learning

Published experiments
Investigating factors affecting the rate of photosynthesis, class practical.
In this experiment the rate of photosynthesis is measured by counting the number of bubbles rising from the cut end of a piece of Elodea or Cabomba .
Lesson organisation
The work could be carried out individually or in groups of up to 3 students (counter, timekeeper and scribe).
Apparatus and Chemicals
Students may choose to use:.
Thermometer, –10 °C –110°C
Coloured filters or light bulbs
Push-button counter
Potassium hydrogencarbonate powder or solution (Hazcard 95C describes this as low hazard)
For each group of students:
Student sheets, 1 per student
Beaker, 600 cm 3 , 1
Metre ruler, 1
Elodea ( Note 1 ) or other oxygenating pond plant ( Note 2 )
Electric lamp
Clamp stand with boss and clamp
Health & Safety and Technical notes
Normal laboratory safety procedures should be followed. There is a slight risk of infection from pond water, so take sensible hygiene precautions, cover cuts and wash hands thoroughly after the work is complete.
Read our standard health & safety guidance
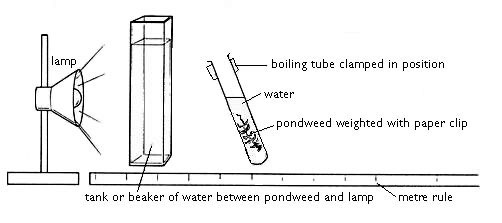
1 Elodea can be stored in a fish tank on a windowsill, in the laboratory or prep room. However it is probably a good idea to replace it every so often with a fresh supply from an aquarist centre or a pond. (It’s worth finding out if any colleague has a pond.) On the day of the experiment, cut 10 cm lengths of Elodea , put a paper-clip on one end to weigh them down and place in a boiling tube of water in a boiling tube rack, near a high intensity lamp, such as a halogen lamp or a fluorescent striplight. Check the Elodea to see if it is bubbling. Sometimes cutting 2–3 mm off the end of the Elodea will induce bubbling from the cut end or change the size of the bubbles being produced.
2 Cabomba (available from pet shops or suppliers of aquaria – used as an oxygenator in tropical fish tanks) can be used as an alternative to Elodea , and some people find it produces more bubbles. It does, though tend to break apart very easily, and fish may eat it very quickly.
3 If possible, provide cardboard to allow students to shield their experiment from other lights in the room.
Ethical issues
Look out for small aquatic invertebrates attached to the pond weed used, and remove them to a pond or aquarium.

Hands-on Activity Bubbling Plants Experiment to Quantify Photosynthesis
Grade Level: 6 (5-7)
Time Required: 1 hour
Expendable Cost/Group: US $3.00
Group Size: 3
Activity Dependency: Do Plants Eat? All About Photosynthesis
Subject Areas: Biology, Life Science
NGSS Performance Expectations:

Activities Associated with this Lesson Units serve as guides to a particular content or subject area. Nested under units are lessons (in purple) and hands-on activities (in blue). Note that not all lessons and activities will exist under a unit, and instead may exist as "standalone" curriculum.
Te newsletter, engineering connection, learning objectives, materials list, more curriculum like this, pre-req knowledge, introduction/motivation, vocabulary/definitions, investigating questions, activity extensions, user comments & tips.

Students perform data analysis and reverse engineering to understand how photosynthesis works. Both are important aspects of being an engineer.
After this activity, students should be able to:
- Explain that photosynthesis is a process that plants use to convert light energy into glucose, a source of stored chemical energy for the plant.
- Describe photosynthesis as a set of chemical reactions in which the plant uses carbon dioxide and water to form glucose and oxygen.
- Describe a simple experiment that provides indirect evidence that photosynthesis is occurring.
- Describe the effects of varying light intensity on the amount of photosynthesis that occurs.
Educational Standards Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards. All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN) , a project of D2L (www.achievementstandards.org). In the ASN, standards are hierarchically structured: first by source; e.g. , by state; within source by type; e.g. , science or mathematics; within type by subtype, then by grade, etc .
Ngss: next generation science standards - science, common core state standards - math.
View aligned curriculum
Do you agree with this alignment? Thanks for your feedback!
International Technology and Engineering Educators Association - Technology
State standards, north carolina - math, north carolina - science.
- 5 liters (about 1¼ gallons) of aged tap water (tap water in an open container that has been allowed to sit for 36-48 hours to eliminate the chlorine used in municipal water supplies)
- 15-20 total Elodea plants; these are hardy freshwater aquarium plants sold in bunches at pet stores and suppliers such as Carolina Biological Supply Company (www.carolina.com)
- string, yarn or twist ties for tying Elodea plants into bunches
- small rocks or similar objects to serve as weights to hold the Elodea plants underwater
- 500-ml beakers, 1 per team
- baking soda, a few tablespoons (sodium bicarbonate)
- timers or watches with second hands, 1 per team
- small adjustable desk lamps that can be set up so that their light bulbs are a few inches above the beakers and shine vertically down onto them; flashlights with strong beams that are mounted on ring stands also work; 1 light source per team
An understanding of photosynthesis, as presented in the associated lesson, Do Plants Eat?
(Get the class' attention and ask them to do as you say.) With one hand, pinch your nose closed. Raise your other hand high in the air. Now take a deep breath and hold it for as long as you can. When you cannot hold your breath any longer, lower your raised hand and unpinch your nose. (Once all hands are down and no one is left holding their breath, move on.) Why did you need to start breathing again? (From their elementary school studies, expect students to be able to tell you that their bodies need air in order to survive.)
What, exactly, is in air? (Students may not know that air contains more than oxygen.) Most of the air we breathe—the atmosphere—consists of nitrogen gas (about 78%). Oxygen is the next largest component (about 21%) and a tiny part (1%) is made up of argon (an inert gas), water vapor and carbon dioxide.
So, specifically what component(s) of air do our bodies need? (Expect them to be able to answer that it is oxygen.) And what do our bodies do with oxygen? That's right, oygen from the air is picked up in the lungs by the blood and carried to all parts of the body, where it is used by muscles and the brain and all the other organs and tissues of the body. We cannot live without it.
From where did the oxygen in the atmosphere come? (They may know or be able to reason that it is the result of all the plants that have lived on the Earth and have been doing photosynthesis for many millions of years.) Today, you will work in teams to conduct an experiment to see if the amount of light plants receive can affect this production of oxygen.
Overall Experiment Plan
- In a class discussion format, students establish a hypothesis to be tested by the class in the experiment.
- Working in teams, students set up and conduct the experiment. Each team conducts two trials: one with the plants lit only by the ambient light available in the classroom when some or all of the room lights are turned off, and one with the plants receiving bright light from the desk lamps. The data collected are the number of bubbles of oxygen that are given off by the plants in a five-minute period, first at low-light levels, and then at high-light levels.
- Then the groups come together to pool their data from each of the two trials. From these data, students individually determine the mean, median and modes for the numbers of bubbles produced during the two different light conditions.
- Then students individually graph the data, using bar graphs that show the mean numbers of bubbles and the ranges for each test condition.
Part 1: Generating a Hypothesis
Explain to the class that before researchers start experiments, they first create a prediction about the expected outcome of the experiment. This prediction is known as a hypothesis. A hypothesis is not simply a guess, however. Instead, it is a prediction based on prior knowledge of or experience with the subject. For example, if a gardener wanted to find out if it was really necessary to fertilize zucchini plants, they might grow 12 zucchini plants, but fertilize only half of them. In this case, the hypothesis being tested might be: Fertilized zucchini plants produce more zucchinis than unfertilized zucchini plants. The data collected to support or refute the hypothesis would be the total number of zucchinis produced by the fertilized plants, compared to the total number produced by the unfertilized plants.
Point out that in the zucchini experiment, the gardener collected data that involved numbers. In science, this is usually the case, because numbers can easily be compared and are cumulative for many things that actually happen, as opposed to things that the experimenter thought might happen.
Then, explain briefly how the photosynthesis experiment will be set up and ask the class to determine a hypothesis to be tested. It shouldn't take them long to come up with a statement such as: The plants that receive more light produce more bubbles than the plants that receive less light.
Part 2: Setting up the Experiment
Perform the following steps with some or all of the classroom lights turned off. Ideally, the room should not be brightly lit, nor should it be dark; adequate light should be present for students to easily see.
- Each team fills a beaker with about 500 ml of aged water for the Elodea. To this water, add a scant one-quarter teaspoon of sodium bicarbonate (baking soda) to provide a source of carbon dioxide for the plants, since they cannot get it from the atmosphere like terrestrial plants do. Stir the water until the sodium bicarbonate is dissolved and the water looks clear.
- Each team obtains enough sections of Elodea plants so that it has about 18-24 inches of total plant length. Arrange them so that all of the plants are at least 1½" under the water in the beaker. Use string or twist ties to hold them together, and then add a small rock to keep the plants from floating to the surface. Point out that the more area exposed to the light above the plant, the more photosynthesis can occur within the leaves. If students form clumps of Elodea, many of leaves will be shaded by those above, and thus may not be able to perform as much photosynthesis. It is best to form the plants into loops that cover the entire bottom of a beaker, instead of a single clump in the middle of the beaker.
Part 3: Running the Experiment
- As soon as the plants are arranged in the beakers, have the team start timing for five minutes. Direct two team members to have their eyes glued to the beaker for those five minutes, watching for bubbles to rise to the water surface. Announce to the third team member the sighting of any bubbles that rise, so s/he can keep count (using tally marks is helpful) and monitor the time, indicating when the five minutes are up. The bubbles are fairly large, about 2 mm in diameter, and so are easily seen when they rise to the surface.
- When all teams have counted bubbles for five minutes (it is quite possible that some teams see no bubbles at all), turn on the room lights and have students position the desk lamps directly above the beakers with the light bulbs only be a few inches above the beakers. Once the lights are in place, have the teams again begin timing and counting/recording bubbles for five minutes.
Part 4: Pooling and Analyzing the Data
- Make a large chart on the classroom board in which teams can fill in the number of bubbles they counted during each of the two light conditions.
- Once the chart is filled in, have students work individually to determine the mean, median, mode and range of each of the two data sets. Allow enough time so that all students arrive at the same answers.
- Provide students with grid paper and direct them to make vertical bar graphs that compare the mean number of bubbles in the two light conditions. Be sure that students include titles, axes labels and legends if different colors are used for the two bars. Then show them how they can indicate the ranges of the data by adding a vertical line segment to the center top of each bar, with the lower end of the line segment situated at the lowest number of bubbles observed by a team, and the upper end of the line segment at the highest number of bubbles observed.
Part 5: Interpreting the Data
- As a class, examine all the data and graphs and revisit the hypothesis. What do these numbers tell us about the amount of photosynthesis that occurred in each of the two light conditions. In other words, was the hypothesis the class tested supported or not?
- Continue with a class discussion to analyse the data. How do you know that the bubbles you saw rise to the surface were bubbles of oxygen? Students may answer that they know photosynthesis produces oxygen, so the bubbles must have been oxygen. However, without a way to determine the chemical composition of the bubbles, it is only an assumption that the bubbles contain oxygen. They might just as well have been bubbles of nitrogen or carbon dioxide, or some other gas from some other process that was occurring in the plants instead of photosynthesis. Nevertheless, since the plants were exposed to light, the bubbles were most likely made of oxygen. Point out that it is important for researchers to make sure they recognize the difference between what they know about an experiment and what they assume about it.
mean: The sum of all the values in a set of data, divided by the number of values in the data set; also known as the average. For example, in a set of five temperature measurements consisting of 22 ºC, 25 ºC, 18 ºC, 22 ºC and 19 ºC, the mean temperature is 106 ºC divided by 5, or 21.2 ºC.
median: Tthe middle value in a set of data, obtained by organizing the data values in an ordered list from smallest to largest, and then finding the value that is at the half-way point in the list. For example, in a set of five temperature measurements consisting of 22º C, 25º C, 18º C, 22 º C, and 19º C, the ordered list of temperatures would be 18º C, 19º C, 22º C, 22º C, and 25º C. The middle value is the third value, 22º C. If the data set consists of an even number of values, the median is determined by averaging the two middle values. For example, in a set of six temperature measurements consisting of 20 ºC, 22 ºC, 25 ºC, 18 ºC, 24 ºC and 19 ºC, the middle values are 20 ºC and 22 ºC. Thus, the median value is the average of 20 ºC and 22 ºC, which is 21 ºC.
mode : The value in a set of data that occurs most frequently. For example, in a set of five temperature measurements consisting of 22 ºC, 25 ºC, 18 ºC, 22 ºC and 19 ºC, the measurement of 22 ºC occurs most frequently, so it is the mode. It is possible to have two or more modes in a set of data, if two or more values occur with equal frequency.
Questions : Evaluate students' comprehension by asking them questions such as:
- What "things" are needed in order for photosynthesis to occur?
- What are the products of photosynthesis?
- Where in the plant does photosynthesis occur?
- Why do plants need water in order to survive?
Graph Analylsis: Provide a graph of data from an experiment similar to the one students just performed, and ask them to draw conclusions from it. For example, the data could represent the heights of corn plants, half of which were grown in the shade of a forest and half of which were grown in an open field.
- What do you think would happen if you left some plants in a completely dark closet for two or three weeks? Why do you think that?
- Why is it important for crop plants to receive enough rainfall?
- The Earth's atmosphere did not always contain as much oxygen as it does now. In fact, at one time it probably contained no oxygen at all. How do you think the oxygen in the Earth's atmosphere got there? Why do you think that?
The light that comes from the sun consists of light waves of many different wavelengths. In the visible spectrum of light, these range from red with the longest wavelength, to violet with the shortest wavelength. Chlorophyll does not respond equally to all wavelengths, or colors of light. Have students use the same experimental setup to determine what color or colors of light result in the most photosynthetic activity. The only modification they need to make is to loosely cover the beaker with colored plastic wrap or cellophane during the five minutes of bubble counting. Since blue wavelengths are the best for most plants, be sure that this is one of the colors available. If possible, have red and one other color available as well.

Through a teacher-led discussion, students realize that the food energy plants obtain comes from sunlight via the plant process of photosynthesis. By counting the number of bubbles that rise to the surface in a five-minute period, students can compare the photosynthetic activity of Elodea in the pre...

Students learn about photosynthesis and cellular respiration at the atomic level and study the basic principles of electromicrobiology—a new field of research that may enable engineers to harness energy at the molecular level.

Contributors
Supporting program, acknowledgements.
This content was developed by the MUSIC (Math Understanding through Science Integrated with Curriculum) Program in the Pratt School of Engineering at Duke University under National Science Foundation GK-12 grant no. DGE 0338262. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: July 12, 2023

The Biology Corner
Biology Teaching Resources

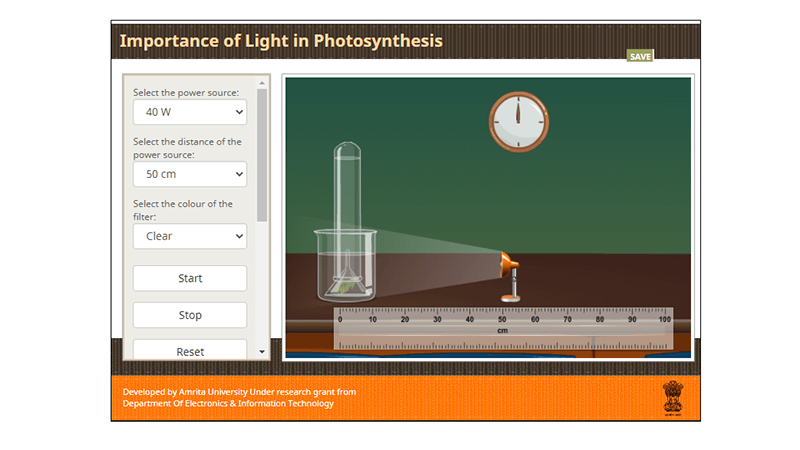
Photosynthesis Virtual Lab

This lab was created to replace the popular waterweed simulator which no longer functions because it is flash-based. In this virtual photosynthesis lab , students can manipulate the light intensity, light color, and distance from the light source.
A plant is shown in a beaker and test tube which bubbles to indicate the rate of photosynthesis. Students can measure the rate over time. There is an included data table for students to type into the simulator, but I prefer to give them their own handout ,
The handout is a paper version for students to write on as the work with the simulator. The document is made with google docs so that it can be shared with remote students.
There are several experiments that can be done in the lab that would complement this virtual experiment. For example, students can use elodea and measure the number of bubbles released when the plant is under a bright light. Algae beads can also be used to measure changes in pH as the plants consume carbon dioxide.
In experiment 2, students specifically look at light color to determine which wavelength of light increases the rate of photosynthesis. Students should discover that green light has a very slow rate. Their collected data is then compared to a graph of the absorption spectrum of light.
Shannan Muskopf
Exploring Our Fluid Earth
Teaching science as inquiry.
- Create new account
- Reset your password
For more option use Advanced Search
Teacher Guide: Elodea Photosynthesis Light
- Elodea sp. (or Egeria sp. often sold under the common name “anacharis”) in a container of fresh water
- Six 50 mL Erlenmeyer flasks
- Six one-hole #2 rubber stoppers
- Six 1 mL graduated glass serological pipettes
- Red and green light filters
- Aluminum foil
- Thermometer
- Fig. 2.40 and 2.41
- Blue light filter (optional)
- Petroleum jelly (optional)
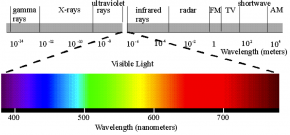
Fig. 2.40. This diagram of the electromagnetic spectrum emphasizes the small portion of the spectrum that is visible to human eyes. Wavelengths are measured in meters (m) along the grey bar and in nanometers (nm) along the colored bar showing visible light.
Image courtesy of Philip Ronan, Wikimedia Commons
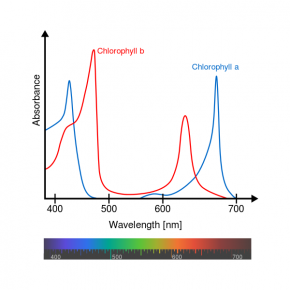
Fig. 2.41. Relative absorbance of the visible wavelengths in sunlight by the pigments chlorophyll a and chlorophyll b
Image courtesy of Daniele Pugliesi and M0tty, Wikimedia Commons
Baking Soda Solution
Baking soda serves as a source of carbon dioxide. The Elodea will be placed in this solution to speed up the photosynthesis reaction .
- Baking soda (sodium bicarbonate, NaHCO 3 )
- Fresh water
- Elodea is a genus of freshwater aquatic plants sold in pet stores for aquariums. Observe and describe the Elodea specimen.
- Use Fig. 2.40 to determine the range of wavelengths that corresponds to white light and to each color: red, blue, and green. Fill in the first column of Table 2.8.
- What color(s) does chlorophyll a absorb most? Least?
- What color(s) does chlorophyll b absorb most? Least?
- Using what you know about the electromagnetic spectrum and chlorophyll, predict the flask that will have the plant with the highest gas production and write “1” in the “Prediction” column of Table 2.8. The more light energy the plant absorbs, the more gas it should produce.
- Write number “2” in the “Prediction” column of Table 2.8 for the flask that you predict will have the second highest gas production.
- Continue numbering in the “Prediction” column of Table 2.8 through “6,” with 6 being the flask that you predict will have the least gas production.
Photosynthesis should be fastest with ____________ wavelength(s) of light and slowest with ____________ wavelength(s) of light because ________________.
- Weigh 25 g of baking soda.
- Add baking soda to 1 L of fresh water.
- Stir until the baking soda is completely dissolved.
- Cut out rectangles of green and red filter paper slightly bigger than the flasks.
- Wrap filters around the flasks, secure with tape. Trim excess filter.
- Wrap one flask with foil.
- Remove a few branches of Elodea from the holding container. Visually inspect the Elodea, and remove any part of the plant that looks unhealthy or has different leaf morphology (shape) than the rest of the plant. Blot Elodea dry with towels.
- Using a balance, weigh 2.5 g of Elodea . The Elodea should be as close as possible to 2.5 g, within 0.1 g (i.e., between 2.4 and 2.6 g). Write the weight in Table 2.8.
- Insert Elodea into the flask. If necessary, you can break the Elodea into smaller pieces and use a skewer to distribute the Elodea evenly in the flask.
- Working over towels, slowly fill each flask all the way to the top with baking soda (sodium bicarbonate) solution.
- Firmly press a stopper into each flask. Sodium bicarbonate solution will spill out of the flask as you insert the stopper. Applying petroleum jelly to the outer surfaces of the rubber stoppers may help to form an airtight seal.
- Insert the pipette into the stopper hole by holding the pipette with a dry towel. Gently twist the tapered tip of the pipette into the stopper until water rises in the pipette and the pipette is firmly in place. The water level should reach between the 0.8 and 0.7 mL lines. DO NOT FORCE the pipette into the stopper as the pipette can break. If you are having difficulty getting the pipette into the stopper, ask your teacher for assistance.
- Dry the outside of each flask with a towel.
- Set the flasks in the sun. Make sure each flask is exposed to a similar amount of sunlight.
- Record the starting volume of the liquid in each pipette and the time in Table 2.8. Read the bottom of the meniscus of the water in the pipette. Note that the numbers on the pipette are smallest at the top and largest at the bottom.
- record the weather conditions, especially noting the amount of sunlight, and
- observe what is happening in the pipettes and the flasks without disturbing the light filters or foil.
- At the end of the experiment, record the ending volume (water level) in each pipette and the time in Table 2.8.
- Subtract the start volume from the final volume to get the total amount of gas produced in each flask. Record the amount in Table 2.8.
- Rank the gas production in each flask, by writing “1” in the “Observation” column for the flask with the plant the produced the most gas and numbering through “6” for the flask that produced the least gas.
- OPTIONAL: repeat procedure steps 7 to 16 using a blue light filter.
- Holding the pipette with a dry towel, gently twist the pipette while pulling up to remove it from the stopper. Stand the pipette upright in a container and let it drip dry.
- Remove the stopper from the flask.
- Dispose of the plants and sodium bicarbonate solution as directed by your teacher. Clean each flask.
- Wipe the light filters with fresh water to remove traces of sodium bicarbonate solution.
- Compare your results to those of other groups using a class data chart.
- What caused the water in the pipettes to rise?
- What gas is being produced in the flasks by Elodea ? What process is producing this gas?
- Compare your predictions and observations. Was your hypothesis supported? Why or why not? Give you answer in terms of absorption of wavelengths by chlorophyll pigments.
- How did your results compare to those of the rest of the class? Hypothesize possible reasons for any unexpected results.
- What gas is likely being produced?
- What process is likely producing this gas?
- What was the purpose of the clear flask with no plant? In other words, is the production of gas the only thing that may affect the starting and ending water volume in the flasks?
- How can you use the results of your control flasks to more accurately calculate gas production in the other flasks?
- How can this experiment be improved to more accurately measure the photosynthesis rate in Elodea ? What sources of error can be further controlled?
- Why are plants green? Answer in terms of the wavelengths of light they absorb. (Hint: What colors do chlorophyll a and chlorophyll b absorb and reflect?)
- Based on Fig. 2.40, if green glass is a green-yellow color, what range of wavelengths might it be reflecting?
- Based on Fig. 2.40 and Fig. 2.41, what color(s) would the pigments chlorophyll a and chlorophyll b appear to the human eye?

Authors & Partners
Partner Organizations

Exploring Our Fluid Earth, a product of the Curriculum Research & Development Group (CRDG), College of Education. © University of Hawai‘i, . This document may be freely reproduced and distributed for non-profit educational purposes.

IMAGES
COMMENTS
In this experiment the rate of photosynthesis is measured by counting the number of bubbles rising from the cut end of a piece of Elodea or Cabomba. Lesson organisation. The work could be carried out individually or in groups of up to 3 students (counter, timekeeper and scribe). Apparatus and Chemicals. Students may choose to use: Hot water. Ice.
Use this classroom dataset from a classic lab to see how light intensity affects photosynthesis. You can also collect, upload, and analyze your own data from this classic biology and life science lab.
Students learn a simple technique for quantifying the amount of photosynthesis that occurs in a given period of time, using a common water plant (Elodea). They use this technique to compare the amounts of photosynthesis that occur under conditions of low and high light levels.
Photosynthesis and Respiration in Elodea. Background Concepts: Plants can carry out both photosynthesis and respiration simultaneously. During photosynthesis, plants are using the energy of the sun to build molecules which effectively store this energy (glucose). Chemically, the photosynthetic reaction looks like this:
In this virtual photosynthesis lab, students can manipulate the light intensity, light color, and distance from the light source. A plant is shown in a beaker and test tube which bubbles to indicate the rate of photosynthesis.
In this experiment you will be investigating the effect of light color (wavelength) on the rate of photosynthesis by measuring gas production in Elodea. Elodea has both chlorophyll a and chlorophyll b .
This lab involves the qualitative measurement of the changes in carbon dioxide concentration associated with respiration and photosynthesis in the freshwater plant Elodea. Bromthymol blue is used as an indicator for the presence of CO2 in solution.
The purpose of this lab is to determine the effects of elodea on CO2 levels and to observe evidence of cellular respiration and photosynthesis. Part 1: Elodea and Bromothymol Blue: 1. Label four test tubes: "control," "3 cm elodea" and "7 cm elodea." and "7 cm dark".
Plants use carbon dioxide and produce oxygen gas during photosynthesis. They produce carbon dioxide during cellular respiration. In this experiment, the student will place aquatic plants under different colors of light in a solution of bromothymol blue.
In this experiment, you will investigate photosynthesis by an aquatic plant, Elodea. Elodea leaves, when placed into water in a light environment, will remove CO 2 from the water and produce glucose by photosynthesis.