

The Impact of Sleep on Learning and Memory
By Kelly Cappello, B.A.
For many students, staying awake all night to study is common practice. According to Medical News Today , around 20 percent of students pull all-nighters at least once a month, and about 35 percent stay up past three in the morning once or more weekly.
That being said, staying up all night to study is one of the worst things students can do for their grades. In October of 2019, two MIT professors found a correlation between sleep and test scores : The less students slept during the semester, the worse their scores.
So, why is it that sleep is so important for test scores? While the answer seems simple, that students simply perform better when they’re not mentally or physically tired, the truth may be far more complicated and interesting.
In the last 20 years, scientists have found that sleep impacts more than just students’ ability to perform well; it improves their ability to learn, memorize, retain, recall, and use their new knowledge to solve problems creatively. All of which contribute to better test scores.
Let’s take a look at some of the most interesting research regarding the impact of sleep on learning and memory.
How does sleep improve the ability to learn?
When learning facts and information, most of what we learn is temporarily stored in a region of the brain called the hippocampus. Some scientists hypothesize that , like most storage centers, the hippocampus has limited storage capacity. This means, if the hippocampus is full, and we try to learn more information, we won’t be able to.
Fortunately, many scientists also hypothesize that sleep, particularly Stages 2 and 3 sleep, plays a role in replenishing our ability to learn. In one study, a group of 44 participants underwent two rigorous sessions of learning, once at noon and again at 6:00 PM. Half of the group was allowed to nap between sessions, while the other half took part in standard activities. The researchers found that the group that napped between learning sessions learned just as easily at 6:00 PM as they did at noon. The group that didn’t nap, however, experienced a significant decrease in learning ability [1].
How does sleep improve the ability to recall information?
Humans have known about the benefits of sleep for memory recall for thousands of years. In fact, the first record of this revelation is from the first century AD. Rhetorician Quintilian stated, “It is a curious fact, of which the reason is not obvious, that the interval of a single night will greatly increase the strength of the memory.”
In the last century, scientists have tested this theory many times, often finding that sleep improves memory retention and recall by between 20 and 40 percent. Recent research has led scientists to hypothesize that Stage 3 (deep non-Rapid Eye Movement sleep, or Slow Wave Sleep) may be especially important for the improvement of memory retention and recall [2].
How does sleep improve long-term memory?
Scientists hypothesize that sleep also plays a major role in forming long-term memories. According to Matthew Walker, professor of neuroscience and psychology at UC Berkeley, MRI scans indicate that the slow brain waves of stage 3 sleep (deep NREM sleep) “serve as a courier service,” transporting memories from the hippocampus to other more permanent storage sites [3].
How does sleep improve the ability to solve problems creatively?
Many tests are designed to assess critical thinking and creative problem-solving skills. Recent research has led scientists to hypothesize that sleep, particularly REM sleep, plays a role in strengthening these skills. In one study, scientists tested the effect of REM sleep on the ability to solve anagram puzzles (word scrambles like “EOUSM” for “MOUSE”), an ability that requires strong creative thinking and problem-solving skills.
In the study, participants solved a couple of anagram puzzles before going to sleep in a sleep laboratory with electrodes placed on their heads. The subjects were woken up four times during the night to solve anagram puzzles, twice during NREM sleep and twice during REM sleep.
The researchers found that when participants were woken up during REM sleep, they could solve 15 to 35 percent more puzzles than they could when woken up from NREM sleep. They also performed 15 to 35 percent better than they did in the middle of the day [4]. It seems that REM sleep may play a major role in improving the ability to solve complex problems.
So, what’s the point?
Sleep research from the last 20 years indicates that sleep does more than simply give students the energy they need to study and perform well on tests. Sleep actually helps students learn, memorize, retain, recall, and use their new knowledge to come up with creative and innovative solutions.
It’s no surprise that the MIT study previously mentioned revealed no improvement in scores for those who only prioritized their sleep the night before a big test. In fact, the MIT researchers concluded that if students want to see an improvement in their test scores, they have to prioritize their sleep during the entire learning process. Staying up late to study just doesn’t pay off.
Interested in learning more about the impact of sleep on learning and memory? Check out this Student Sleep Guide .
Author Biography
Kelly Cappello graduated from East Stroudsburg University of Pennsylvania with a B.A. in Interdisciplinary Studies in 2015. She is now a writer, specialized in researching complex topics and writing about them in simple English. She currently writes for Recharge.Energy , a company dedicated to helping the public improve their sleep and improve their lives.
- Mander, Bryce A., et al. “Wake Deterioration and Sleep Restoration of Human Learning.” Current Biology, vol. 21, no. 5, 2011, doi:10.1016/j.cub.2011.01.019.
- Walker M. P. (2009). The role of slow wave sleep in memory processing. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine, 5(2 Suppl), S20–S26.
- Walker, Matthew. Why We Sleep. Scribner, 2017.
- Walker, Matthew P, et al. “Cognitive Flexibility across the Sleep–Wake Cycle: REM-Sleep Enhancement of Anagram Problem Solving.” Cognitive Brain Research, vol. 14, no. 3, 2002, pp. 317–324., doi:10.1016/s0926-6410(02)00134-9.
Posted on Dec 21, 2020 | Tagged: learning and memory
© The Trustees of the University of Pennsylvania | Site best viewed in a supported browser . | Report Accessibility Issues and Get Help | Privacy Policy | Site Design: PMACS Web Team. | Sitemap
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Sleep deprivation: Impact on cognitive performance
Paula alhola, päivi polo-kantola.
- Author information
- Copyright and License information
Correspondence: Paula Alhola, Department of Psychology, University of Turku, FI-20014 Turku, Finland, Email [email protected]
Today, prolonged wakefulness is a widespread phenomenon. Nevertheless, in the field of sleep and wakefulness, several unanswered questions remain. Prolonged wakefulness can be due to acute total sleep deprivation (SD) or to chronic partial sleep restriction. Although the latter is more common in everyday life, the effects of total SD have been examined more thoroughly. Both total and partial SD induce adverse changes in cognitive performance. First and foremost, total SD impairs attention and working memory, but it also affects other functions, such as long-term memory and decision-making. Partial SD is found to influence attention, especially vigilance. Studies on its effects on more demanding cognitive functions are lacking. Coping with SD depends on several factors, especially aging and gender. Also interindividual differences in responses are substantial. In addition to coping with SD, recovering from it also deserves attention. Cognitive recovery processes, although insufficiently studied, seem to be more demanding in partial sleep restriction than in total SD.
Keywords: Sleep deprivation, cognitive performance, sleep restriction, recovery, aging, gender differences
Introduction
A person’s quality of life can be disrupted due to many different reasons. One important yet underestimated cause for that is sleep loss ( National Sleep Foundation 2007 ). Working hours are constantly increasing along with an emphasis on active leisure. In certain jobs, people face sleep restriction. Some professions such as health care, security and transportation require working at night. In such fields, the effect of acute total sleep deprivation (SD) on performance is crucial. Furthermore, people tend to stretch their capacity and compromise their nightly sleep, thus becoming chronically sleep deprived.
When considering the effects of sleep loss, the distinction between total and partial SD is important. Although both conditions induce several negative effects including impairments in cognitive performance, the underlying mechanisms seem to be somewhat different. Particularly, results on the recovery from SD have suggested different physiological processes. In this review, we separately consider the effects of acute total and chronic partial SD and describe the effects on cognitive performance. The emphasis on acute total SD reflects the quantity of studies carried out compared with partial SD. The effects of aging and gender, as well as interindividual differences are discussed. We concentrate on the studies that have been published since 1990.
Sleep and sleep loss
The need for sleep varies considerably between individuals ( Shneerson 2000 ). The average sleep length is between 7 and 8.5 h per day ( Kripke et al 2002 ; Carskadon and Dement 2005 ; Kronholm et al 2006 ). Sleep is regulated by two processes: a homeostatic process S and circadian process C (eg, Achermann 2004 ). The homeostatic process S depends on sleep and wakefulness; the need for sleep increases as wakefulness continues. The theory for circadian process C suggests a control of an endogenous circadian pacemaker, which affects thresholds for the onset and offset of a sleep episode. The interaction of these two processes determines the sleep/wake cycle and can be used to describe fluctuations in alertness and vigilance. Although revised “three-process models” (eg, Akerstedt and Folkard 1995 ; Van Dongen et al 2003b ; Achermann 2004 ) have been suggested, this classical model is the principal one used for study designs in SD research.
There are many unanswered questions regarding both the functions of sleep and the effects of sleep loss. Sleep is considered to be important to body restitution, like energy conservation, thermoregulation, and tissue recovery ( Maquet 2001 ). In addition, sleep is essential for cognitive performance, especially memory consolidation ( Maquet 2001 ; Stickgold 2005 ). Sleep loss, instead, seems to activate the sympathetic nervous system, which can lead to a rise of blood pressure ( Ogawa et al 2003 ) and an increase in cortisol secretion ( Spiegel et al 1999 ; Lac and Chamoux 2003 ). Immune response may be impaired and metabolic changes such as insulin resistance may occur (for review, see Spiegel et al 2005 ). People who are exposed to sleep loss usually experience a decline in cognitive performance and changes in mood (for meta-analyses, see Pilcher and Huffcutt 1996 ; Philibert 2005 ).
Sleep deprivation is a study design to assess the effects of sleep loss. In acute total SD protocols, the subjects are kept awake continuously, generally for 24–72 hours. In chronic partial SD, subjects are allowed restricted sleep time during several consecutive nights. Although chronic sleep restriction is more common in the normal population and thus offers a more accurate depiction of real life conditions, total SD has been more thoroughly explored.
Cognitive performances measured in SD studies have included several domains. The most thoroughly evaluated performances include different attentional functions, working memory, and long-term memory. Visuomotor and verbal functions as well as decision-making have also been assessed. Sleep deprivation effects on cognitive performance depend on the type of task or the modality it occupies (eg, verbal, visual, or auditory). In addition, task demands and time on task may play a role. The task characteristics are discussed in more detail in following sections where the existing literature on the cognitive effects of SD is reviewed.
Mechanisms behind sleep loss effects
Some hypotheses are proposed to explain why cognitive performance is vulnerable to prolonged wakefulness. The theories can be divided roughly in two main approaches, in which SD is assumed to have (1) general effects on alertness and attention, or (2) selective effects on certain brain structures and functions. In addition, individual differences in the effects have been reported.
The general explanation relies on the two-process model of sleep regulation. Cognitive impairments would be mediated through decreased alertness and attention through lapses, slowed responses, and wake-state instability. Attentional lapses, brief moments of inattentiveness, have been considered the main reason for the decrease in cognitive performance during sleep deprivation (on lapse hypothesis, eg, Williams et al 1959, see Dorrian et al 2005 ; Kjellberg 1977 ). The lapses are caused by microsleeps characterized by very short periods of sleep-like electro-encephalography (EEG) activity ( Priest et al 2001 ). Originally, it was thought that in between the lapses, cognitive performance almost remained intact, but the slowing of cognitive processing has also been observed independent of lapsing ( Kjellberg 1977 ; Dorrian et al 2005 ). According to these hypotheses, performance during SD would most likely deteriorate in long, simple, and monotonous tasks requiring reaction speed or vigilance. In addition to the lapses and response slowing, considerable fluctuations in alertness and effort have been observed during SD. According to the wake-state instability hypothesis, those fluctuations lead to variation in performance ( Doran et al 2001 ).
According to explanations on selective impact, SD interferes with the functioning of certain brain areas and thus impairs cognitive performance. This approach is also referred to as the ‘sleep-based neuropsychological perspective’ ( Babkoff et al 2005 ). Perhaps the most famous theory in this category is the prefrontal vulnerability hypothesis, first proposed by Horne (1993) . It suggests that SD especially impairs cognitive performances that depend on the prefrontal cortex. These include higher functions, such as language, executive functions, divergent thinking, and creativity. In order to show the SD effect, the tests should be complex, new, and interesting. A good performance would require cognitive flexibility and spontaneity. This theory also assumes that the deterioration of subjects’ performance in simple and long tasks is merely due to boredom ( Harrison and Horne 1998 ; Harrison and Horne 1999 ; Harrison and Horne 2000 ). The specific brain areas that are vulnerable to sleep loss have been explored using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET). Those studies, however, have mainly measured working memory or other attentional functions with the type of tasks that are not traditionally emphasized in the prefrontal vulnerability hypothesis (for summary, see Chee et al 2006 ).
Individuals differ in terms of the length, timing, and structure of sleep. Therefore, it is logical to hypothesize that interindividual differences are also important in reaction to SD. Studies have consistently found that some people are more vulnerable to sleep loss than others (for review, see Van Dongen et al 2005 ). In reference to trait differential vulnerability to SD, Van Dongen et al (2005) have proposed the concept of the “trototype”, as compared to the terms “chronotype” and “somnotype”, which define interindividual differences in the timing of circadian rhythmicity and sleep duration. Since a comprehensive review of the interindividual differences in sleep and performance has been published recently ( Van Dongen et al 2005 ), we will focus here on the studies with group comparisons and just briefly address the trait-like vulnerability.
Acute total sleep deprivation
Attention and working memory.
The two most widely studied cognitive domains in SD research are attention and working memory, which in fact are interrelated. Working memory can be divided into four subsystems: phonological loop, visuospatial sketchpad, episodic buffer and central executive ( Baddeley and Hitch 1974 ; Baddeley 2000 ). The phonological loop is assumed to temporarily store verbal and acoustic information (echo memory); the sketchpad, to hold visuospatial information (iconic memory), and the episodic buffer to integrate information from several different sources. The central executive controls them all. Executive processes of working memory play a role in certain attentional functions, such as sustained attention ( Baddeley et al 1999 ), which is referred to here as vigilance. Both attention and working memory are linked to the functioning of frontal lobes (for a review, see Naghavi and Nyberg 2005 ). Since the frontal brain areas are vulnerable to SD ( Harrison et al 2000 ; Thomas et al 2000 ), it can be hypothesized that both attention and working memory are impaired during prolonged wakefulness.
The decrease in attention and working memory due to SD is well established. Vigilance is especially impaired, but a decline is also observed in several other attentional tasks ( Table 1 ). These include measures of auditory and visuo-spatial attention, serial addition and subtraction tasks, and different reaction time tasks ( Table 1 ). The most frequently used task is the psychomotor vigilance test (PVT, lasts usually 10 min) ( Dinges and Powell 1985 ), which is sensitive to sleep loss effects and provides information about both reaction speed and lapses. In working memory, the tests have varied from n-back style tasks with different demand levels to choice-reaction time tasks with a working memory component ( Table 1 ). However, some studies have also failed to find any effect. After one night of SD, no difference was observed between deprived and non-deprived subjects in simple reaction time, vigilance, or selective attention tasks in one study ( Forest and Godbout 2000 ). Performance on the Wisconsin Card Sorting Test, a measure of frontal lobe function, also remained even ( Binks et al 1999 ; Forest and Godbout 2000 ). These results may be partly biased because of small sample sizes, inadequate control of the subjects’ sleep history or the use of stimulants before the study.
Cognitive tests in which deterioration of performance has been reported during acute total sleep deprivation
Abbreviations: SD, sleep deprivation; WAIS, Wechsler Adult Intelligence Scale; WAIS-R, Wechsler Adult Intelligence Scale-Revised.
Outcomes are inconsistent in various dual tasks used for measuring divided attention. Sleep deprivation of 24 h impaired performance in one study ( Wright and Badia 1999 ), whereas in two others, performance was maintained after 25–35 h of SD ( Drummond et al 2001 ; Alhola et al 2005 ). The divergent findings in these studies may be explained by the uneven loads between different subtests as well as by uncontrolled practice effect. Although dividing attention between different tasks puts high demands on cognitive capacity, subjects often attempt to reduce the load by automating some easier procedures of a dual or multitask. In the study by Wright and Badia (1999) , the test was not described; in the study by Alhola et al (2005) , subjects had to count backwards and carry out a visual search task simultaneously, and in the study by Drummond et al (2001) subjects had to memorize words and complete a serial subtraction task sequentially. In addition, differences in essential study elements, like the age and gender of participants, as well as the duration of SD, further complicate comparison of the results.
In the tasks measuring attention or working memory, two aspects of performance are important: speed and accuracy. In practice, people can switch their emphasis between the two with attentional focusing ( Rinkenauer et al 2004 ). Oftentimes, concentrating on improving one aspect leads to the deterioration of the other. This is called the speed/accuracy trade-off phenomenon. Some SD studies have found impairment only in performance speed, whereas accuracy has remained intact ( De Gennaro et al 2001 ; Chee and Choo 2004 ). In others, the results are the opposite ( Kim et al 2001 ; Gosselin et al 2005 ). De Gennaro et al (2001) proposed that in self-paced tasks, there is likely to be a stronger negative impact on speed, while accuracy remains intact. In experimenter-paced tasks, the effect would be the opposite. However, many studies show detrimental effect on both speed and accuracy (eg, Smith et al 2002 ; Jennings et al 2003 ; Chee and Choo 2004 ; Habeck et al 2004 ; Choo et al 2005 ). The speed/accuracy trade-off phenomenon is moderately affected by gender, age, and individual differences in response style ( Blatter et al 2006 ; Karakorpi et al 2006 ), which could be a reason for inconsistencies in the SD results. It has been argued that low signal rates increase fatigue during performance in SD studies and that subjects may even fall asleep during the test ( Dorrian et al 2005 ). Therefore, tasks with different signal loads may produce different results in terms of performance speed and accuracy.
Long-term memory
Long-term memory can be divided between declarative and non-declarative (procedural) memory. Declarative memory is explicit and limited, whereas non-declarative memory is implicit and has a practically unlimited capacity. Declarative memory includes semantic memory, which consists of knowledge about the world, and episodic memory, which holds autobiographical information. The contents of declarative memory can be stored in visual or verbal forms and they can be voluntarily recalled. Non-declarative or procedural memory includes the information needed in everyday functioning and behavior, eg, motor and perceptual skills, conditioned functions and priming. In previous studies, long-term memory has been measured with a variety of tasks, and the results are somewhat inconsistent.
In verbal episodic memory, SD of 35 h impaired free recall, but not recognition ( Drummond et al 2000 ). The opposite results were obtained with one night of SD ( Forest and Godbout 2000 ). The groups in both studies were quite small (in Drummond’s study, N = 13; in Forest and Godbout’s study, experimental group = 9, control group = 9), which offers a possible explanation for the variation in results. In addition, Drummond et al (2000) used a within-subject design, whereas Forest and Godbout (2000) had a between-subject design. In visual memory, recognition was similar in the experimental and control groups when the measurement was taken once after 36 h SD ( Harrison and Horne 2000 ), whereas the practice effect in visual recall was postponed by SD in a study with three measurements (baseline, 25 h SD, recovery; Alhola et al 2005 ). Performance was impaired in probed forced memory recall ( Wright and Badia 1999 ), and memory search ( McCarthy and Waters 1997 ), but no effect was found in episodic memory ( Nilsson et al 2005 ), implicit memory, prose recall, crystallized semantic memory, procedural memory, or face memory ( Quigley et al 2000 ). In the studies failing to find an effect, however, the subjects spent only the SD night under controlled conditions ( Quigley et al 2000 ; Nilsson et al 2005 ).
Free recall and recognition are both episodic memory functions which seem to be affected differently by SD. Temporal memory for faces (recall) deteriorated during 36 h of SD, although in the same study, face recognition remained intact ( Harrison and Horne 2000 ). In verbal memory, the same pattern was observed ( Drummond et al 2000 ). One explanation may be different neural bases, which supports the prefrontal vulnerability hypothesis. Episodic memory is strongly associated with the functioning of the medial temporal lobes ( Scoville and Milner 2000 ), but during free recall in a rested state, even stronger brain activation is found in the prefrontal cortex ( Hwang and Golby 2006 ). It is unclear whether this prefrontal activation reflects episodic memory function, the organization of information in working memory, or the executive control of attention and memory. Recognition, instead, presumably relies on the thalamus in addition to medial temporal lobes ( Hwang and Golby 2006 ). Since SD especially disturbs the functioning of frontal brain areas ( Drummond et al 1999 ; Thomas et al 2000 ), it is not surprising that free recall is more affected than recognition.
Although the prefrontal cortex vulnerability hypothesis has received wide support in the field of SD research, other brain areas are also involved. For instance, the exact role of the thalamus remains unknown. Some studies measuring attention or working memory have noted an increase in thalamic activation during SD (eg, Portas et al 1998 ; Chee and Choo 2004 ; Habeck et al 2004 ; Choo et al 2005 ). This may reflect an increase in phasic arousal or an attempt to compensate attentional performance during a demanding condition of low arousal caused by SD ( Coull et al 2004 ). In other cognitive tasks such as verbal memory ( Drummond and Brown 2001 ) or logical reasoning ( Drummond et al 2004 ), no increase in thalamic activation was found despite the fact that behavioral deterioration occurred. This implies that thalamic activation during SD is mainly related to some attentional function or compensation, providing further support for the hypothesis that “prefrontal dependent” recall is more affected by SD than “thalamus dependent” recognition. However, it is possible that the brain activation patterns during SD reflect something more than merely different cognitive domains. Harrison and Horne (2000) stated that their results may also reflect the difficulty of the task assigned to subjects.
Other cognitive functions
Sleep deprivation impairs visuomotor performance, which is measured with tasks of digit symbol substitution, letter cancellation, trail-making or maze tracing ( Table 1 ). It is believed that visual tasks would be especially vulnerable to sleep loss because iconic memory has short duration and limited capacity ( Raidy and Scharff 2005 ). Another suggestion is that SD impedes engagement of spatial attention, which can be observed as impairments in saccadic eye movements ( Bocca and Denise 2006 ). Decreased oculomotor functioning is associated with impaired visual performance ( De Gennaro et al 2001 ) and sleepiness (eg, De Gennaro et al 2001 ; Zils et al 2005 ). However, further research is needed to confirm this explanation, since not all studies have found oculomotor impairment with cognitive performance decrements ( Quigley et al 2000 ).
Reasoning ability during SD has for the most part been measured with Baddeley’s logical reasoning task or its modified versions. Again the results are inconsistent (deteriorated performance was reported by Blagrove et al 1995 ; McCarthy and Waters 1997 ; Monk and Carrier 1997 , and Harrison and Horne 1999 ; no effects were noted by Linde and Bergstrom 1992 ; Quigley et al 2000 , or Drummond et al 2004 ). The studies reporting no effect have mainly used SD of ca. 24 h ( Linde and Bergström 1992 ; Quigley et al 2000 ), whereas in the studies showing an adverse effect, the SD period has been longer (36 h). Thus reasoning ability seems to be maintained during short-term SD. However, choosing divergent study designs may result in different outcomes. Monk and Carrier (1997) repeated the cognitive test every 2 h and found deterioration after as little as 16 h of SD. In the studies with zero-results, cognitive tests were carried out in the morning ( Linde and Bergström 1992 ; Quigley et al 2000 ) or the practice effect was not adequately controlled ( Drummond et al 2004 ). In the studies with longer SD, the tests have been conducted either in the late afternoon ( McCarthy and Waters 1997 ; Harrison and Horne 1999 ) or have been repeated several times ( Blagrove et al 1995 ; Monk and Carrier 1997 ). Therefore, the different results may reflect the effect of circadian rhythm on alertness and cognitive performance. In the morning or before noon, the circadian process reaches its peak, inducing greater alertness, whereas the timing of the circadian nadir coincides with the late afternoon testing (see Achermann 2004 ).
In addition to the cognitive domains already introduced, total SD affects several other cognitive processes as well. It increases rigid thinking, perseveration errors, and difficulties in utilizing new information in complex tasks requiring innovative decision-making ( Harrison and Horne 1999 ). Deterioration in decision-making also appears as more variable performance and applied strategies ( Linde et al 1999 ), as well as more risky behavior ( Killgore et al 2006 ). Several other tasks have been used in the sleep deprivation studies ( Table 1 ). For example, motor function, rhythm, receptive and expressive speech, and memory measured with the Luria-Nebraska Neuropsychological Battery deteriorated after one night of SD, whereas tactile function, reading, writing, arithmetic and intellectual processes remain intact ( Kim et al 2001 ).
The adverse effects of total SD shown in experimental designs have also been confirmed in real-life settings, mainly among health care workers, professional drivers and military personnel ( Samkoff and Jacques 1991 ; Otmani et al 2005 ; Philibert 2005 ; Russo et al 2005 ). Performance of residents in routine practice and repetitive tasks requiring vigilance becomes more error-prone when wakefulness is prolonged (for a review, see Samkoff and Jacques 1991 ). However, in new situations or emergencies, the residents seem to be able to mobilize additional energy sources to compensate for the effects of tiredness. More recent meta-analysis shows that SD of less than 30 h causes a significant decrease in both the clinical and overall performance of both residents and non-physicians ( Philibert 2005 ).
What role does motivation play in cognitive performance? Can high motivation reverse the adverse effect of SD? Does poor motivation further deteriorate performance? According to a commonly held opinion, high motivation compensates for a decrease in performance, but only a few attempts have been made to confirm this theory. Estimating the compensatory effect of motivation in performance during SD is generally difficult, because persons participating in research protocols, especially in SD studies, usually have high initial motivation. The concept of motivation is closely linked to the “attentional effort” that is considered a cognitive incentive (for a review, see Sarter et al 2006 ). According to Sarter et al (2006) , “increases in attentional effort do not represent primarily a function of task demands but of subjects’ motivation to perform.” Furthermore, attentional effort is a function of explicit and implicit motivational forces and may be increased especially when the subjects are motivated or when they detect signals of performance decrements ( Sarter et al 2006 ).
Harrison and Horne (1998 , 1999) suggest that the deterioration of cognitive performance during SD could be due to boredom and lack of motivation caused by repeated tasks, especially if the tests are simple and monotonous. They used short, novel, and interesting tasks to abolish this motivational gap, yet still noted that SD impaired performance. In contrast, other researchers suggest that sleep-deprived subjects could maintain performance in short tasks by being able to temporarily increase their attentional effort. When a task is longer, performance deteriorates as a function of time. A meta-analysis by Pilcher and Huffcutt (1996) provides support for that: total SD of less than 45 h deteriorated performance more severely in complex tasks with a long duration than in simple and short tasks. Based on this, it is probably necessary to make a distinction between mere attentional effort and more general motivation. Although attentional effort reflects motivational aspects in performance, motivation in a broader sense can be considered a long-term process such as achieving a previously set goal, eg, completing a study protocol. If one has already invested a great deal of time and effort in the participation, motivation to follow through may be increased.
Different aspects of motivation were investigated in a study with 72 h SD, where the subjects evaluated both motivation to perform the tasks and motivation to carry out leisure activities ( Mikulincer et al 1989 ). Cognitive tasks were repeated every two hours. Performance motivation decreased only during the second night of SD, whereas leisure motivation decreased from the second day until the end of the study on the third day. The authors concluded that the subjects were more motivated to complete experimental testing than to enjoy leisure activities because by performing the tasks, they could advance the completion of the study. The researchers suggested that the increased motivation towards the tasks on the third day reflected the “end spurt effect” caused by the anticipation of sleep.
Providing the subjects with feedback on their performance or rewarding them for effort or good performance is shown to help maintain performance both in normal, non-deprived conditions ( Tomporowski and Tinsley 1996 ) and during SD ( Horne and Pettitt 1985 ; Steyvers 1987 ; Steyvers and Gaillard 1993 ). In a large study with 61 subjects (experimental group = 29), with SD of 34–36 h, and with a comprehensive test battery, the subjects were continuously encouraged and provided with 2–3 minute breaks between the tests ( Binks et al 1999 ). Furthermore, they were told they would receive a monetary award for completing all tests with “honest effort”. As result, no deteriorating effect on cognitive performance was found. Unfortunately, a non-motivated control group was not included and thus the effect of motivation remained uncertain. In general, since this issue has not been addressed sufficiently, it is difficult to specify the role of motivation in performance. It seems that motivation affects performance, but it also appears that SD can lead to a loss of motivation.
Self-evaluation of cognitive performance
It has been suggested that the self-evaluation of cognitive performance is impaired by SD. During 36 h SD, the subjects became more confident that their answers were correct as the wakefulness continued ( Harrison and Horne 2000 ). Confidence was even stronger when the answer was actually wrong. In another study, performance was similar between sleep-deprived and control groups in several attentional assessments, but the deprived subjects evaluated their performance as moderately impaired ( Binks et al 1999 ). The controls considered that their performance was high.
The ability to evaluate one’s own cognitive performance depends on age and on the study design. Young people seem to underestimate the effect of SD, whereas older people seem to overestimate it. In a simple reaction time task, both young (aged 20–25 years) and aging (aged 52–63 years) subjects considered that their performance had deteriorated after 24 h SD, although performance was actually impaired only in young subjects ( Philip et al 2004 ). When it comes to the study design and methodology, the way in which the self-evaluation is done may affect the outcome. The answers possibly reflect presuppositions of the subjects or their desire to please the researcher. The repetition of tasks is also essential. Evaluation ability is poor in studies with one measurement only ( Binks et al 1999 ; Harrison and Horne 2000 ; Philip et al 2004 ), whereas in repeated measures, the subjects are shown to be able to assess their performance quite reliably during 60–64 h SD and recovery ( Baranski et al 1994 ; Baranski and Pigeau 1997 ). Thus, self-evaluation is likely to be more accurate when subjects can compare their performance with baseline.
Chronic partial sleep restriction
Although chronic partial sleep restriction is common in everyday life and even more prevalent than total SD, surprisingly few studies have evaluated its effects on cognitive performance. Even fewer studies have compared the effects of acute total sleep deprivation and chronic partial sleep restriction. Belenky and co-workers (2003) evaluated the effect of partial sleep restriction in a laboratory setting in groups which were allowed to spend 3, 5 or 7 h in bed daily for seven consecutive days. The control group spent 9 h in bed. In the 3 h group, both speed and accuracy in the PVT deteriorated almost linearly as the sleep restriction continued. In this group, performance was clearly the worst. In the 5- and 7 h groups, performance speed deteriorated after the first two restriction nights, but then remained stable (though impaired) during the rest of the sleep restriction from the third night onwards. Impairment was greater in the 5- than 7 h group. Accuracy followed the same pattern in the 7 h group, but further declined in the 5 h group as the study went on. The control group’s performance did not change during the study. Intriguingly, a highly similar pattern was observed in another study with the same task when sleep was restricted by 33% of the subject’s habitual nightly sleep, which resulted in 5 h of sleep per night on average ( Dinges et al 1997 ). Both speed and accuracy were impaired at the beginning of the sleep restriction period followed by a plateau and finally, another drop after the seventh night of deprivation. However, no change was found in probed recall memory or serial addition tests, probably because of the practice effect and short duration of the tests (serial addition test: 1 min).
It is difficult to compare the effects of total and partial SD based on existing literature due to large variation in methodologies, including the length of SD or the type of cognitive measures. The only study that has compared total and partial SD found that after controlling learning effects, cognitive performance declined almost linearly in the course of the study in all four experimental groups ( Van Dongen et al 2003a ): one group was exposed to 3 nights total SD, and in other experimental groups, time in bed was restricted to 4 or 6 h for 14 consecutive days. The control group was allowed 8 h in bed for 14 days. Impairment in psychomotor vigilance test and digit symbol substitution task for the 4 h group after 14 days was equal to that of the total SD group after 2 nights. Deterioration in the serial addition/subtraction task for the 4 h group was similar to that of the total SD group after 1 night. The effect of 6 h restricted sleep corresponded to 1 night of total SD in psychomotor vigilance and digit symbol. Performance remained unaffected in the control group.
According to the well-controlled studies ( Dinges et al 1997 ; Belenky et al 2003 ; Van Dongen et al 2003a ), the less sleep obtained due to sleep restriction, the more cognitive performance is impaired. Otherwise, it is difficult to draw conclusions about the effects of chronic sleep restriction because of methodological problems in the previous studies. Blagrove et al (1995) compared subjects that slept at home either 5 h or 8 h per night for 4 weeks and found no effect in a short task of logical reasoning (duration 5 min). The statistical analyses were compromised by the small sample size (6 subjects in the experimental group and only 4 subjects in the control group). In another protocol, they also carried out auditory vigilance test, two column addition, finding embedded figures, and logical reasoning (10 min) tasks, and again no effect was observed with groups of 6–8 subjects having 4, 5 or 8 h sleep per night for 7, 19 or 40 weeks respectively ( Blagrove et al 1995 ). Casement et al (2006) reported no change in working memory and motor speed in the group whose sleep was restricted to 4 h per night for 9 nights. In the control group, performance improved. The study was carried out in a controlled clinical environment, but only one short test session per day was included, which means that subjects may have been able to temporarily increase their effort and thus maintain their performance. Furthermore, the results were confounded by the practice effect. In other sleep restriction studies, SD cannot be considered chronic, since the length of the restriction has been 1–3 nights ( Stenuit and Kerkhofs 2005 ; Swann et al 2006 ; Versace et al 2006 ).
Since chronic partial SD mimics every day life situations more than acute total SD, additional studies on how it affects cognitive performance are warranted. In addition, the tasks used in previous studies have been quite short and simple, and trials with more demanding cognitive tasks are required. The effects of sleep restriction have also been addressed by drive simulation studies, which are interesting and practical designs. Just one night of restricted sleep (4 h) increased right edge-line crossings in a motorway drive simulation of 90 minutes ( Otmani et al 2005 ). However, neither the drivers’ position in the lane nor the amplitude and frequency of steering wheel movements were affected. One sleep-restricted night did not increase the probability of a crash, but after five nights of partial SD, the quantity of accidents increased ( Thorne et al 1999 ).
Cognitive recovering from sleep deprivation
The recovery processes of cognitive performance after sleep loss are still obscure. In many SD studies, the recovery period has either not been included in the protocol or was not reported. Recovery sleep is distinct from normal sleep. Sleep latency is shorter, sleep efficiency is higher, the amounts of SWS and REM-sleep are increased and percentages of stage 1 sleep and awake are decreased ( Armitage et al 2001 ; Kilduff et al 2005 ). The characteristics of recovery sleep may also depend on circumstances and some differences seem to come with eg, aging ( Kalleinen et al 2006 ). Evidence suggests that one sleep period (at least eight hours) can reverse the adverse effects of total SD on cognition ( Brendel et al 1990 ; Corsi-Cabrera et al 2003 ; Adam et al 2006 ; Drummond et al 2006 ; Kendall et al 2006 ). The tasks have been mainly simple attentional tasks; for example, the PVT used by Adam et al (2006) has been proven to have practically no learning curve and little if any correlation with aptitude ( Durmer and Dinges 2005 ). Thus, it is likely that the improvement was mostly caused by the recovery process and not just the practice effect.
After chronic partial sleep restriction, the recovery process of cognitive functioning seems to take longer than after acute total SD. Performance in the PVT was not restored after one 10 h recovery night, but approached the baseline level after two 10 h nights in a study with seven consecutive sleep restriction nights with 5 h sleep/night ( Dinges et al 1997 ). Using the same test, three 8 h recovery nights were not enough to restore performance after one week of sleep restriction even in the group that spent 7 h time in bed (the study is explained in greater detail in paragraph 1 of “Partial sleep restriction”, Belenky et al 2003 ). The group that spent 3 h in bed showed the greatest decline as well as the greatest recovery, although it did not reach baseline level again. In the 5 h group, a similar deterioration-recovery curve was observed, although it was not as steep. Those authors concluded that during mild and moderate chronic partial SD, the brain adapted to a stressful condition to maintain performance, yet at a reduced level. This adaptation process was obviously so demanding that it postponed the restoration of normal functioning. According to their results, it could be further interpreted that when sleep restriction was severe, no such adaptation occurred, which in turn allowed for greater recovery. However, these results may be biased because of poor statistical sensitivity in multiple comparisons. They have also been criticized by eg, Van Dongen et al (2004) , who pointed out that another confounding factor may have been considerable interindividual differences in recovery rates. Since interindividual differences have been observed in response to SD, it is likely – although not yet adequately verified – that those individual traits also affect the recuperation.
Sleep deprivation in different populations
Sleep structure changes with aging. Slow wave sleep and sleep efficiency decrease, and alterations in the circadian rhythm occur (for reviews, see Dzaja et al 2005 ; Gaudreau et al 2005 ). Sleep complaints also become more frequent ( Leger et al 2000 ). Yet, during prolonged wakefulness, cognitive performance seems to be maintained better in aging people than in younger ones ( Bonnet and Rosa 1987 ; Smulders et al 1997 ; Philip et al 2004 ; Stenuit and Kerkhofs 2005 ). Total SD of 24 h deteriorated vigilance in young subjects (20–25 years), whereas performance in aging subjects (52–63 years) remained unaffected ( Philip et al 2004 ). Similarly, during three consecutive nights of partial SD (4 h in bed) performance in psychomotor vigilance task declined more in young subjects (20–30 years) than in aging ones (55–65 years, Stenuit and Kerkhofs 2005 ). In visual episodic memory, visuomotor performance and divided attention, aging subjects (58–72 years) were able to maintain their performance after 25 h of SD and showed improvement only after a recovery night ( Alhola et al 2005 ). However, no comparison with young subjects was made in that study.
Sleep deprivation deteriorates accuracy of performance, especially in young subjects ( Brendel et al 1990 ; Smulders et al 1997 ; Adam et al 2006 ; Karakorpi et al 2006 ). Regarding performance speed, however, results have been inconsistent and the performance of aging subjects has declined more, less, or equally compared to that of younger people. In simple and two-choice reaction time tasks as well as in a vigilance task, reaction speed was impaired in aging subjects (59–72 years) during 40 h SD, whereas young subjects (20–26 years) kept up their speed ( Karakorpi et al 2006 ). These results followed the speed/accuracy trade-off phenomenon so that aging subjects maintained accuracy at the expense of speed and the younger ones did the opposite. In contrast, two other studies found that young subjects were slower than aging subjects ( Brendel et al 1990 ; Adam et al 2006 ). During 24 h wakefulness, performance speed in a vigilance task was impaired in both 20- and 80-year-olds, but more so in the young subjects ( Brendel et al 1990 ). This was confirmed in another study with 40 h SD ( Adam et al 2006 ). When measuring reaction speed in three different choice-reaction time tasks, performance deteriorated similarly in young (18–24 years) and aging (62–73 years) subjects after 28 h total SD ( Smulders et al 1997 ).
Even though there is some evidence that older subjects tolerate SD better than young subjects, it is difficult to determine the age effect during SD with precision. However, because of age-related changes in many aspects of sleep and wakefulness, it is plausible that aging influences reactions to SD. As suggested previously, the weaker SD effect in aging may be due to attenuation of the circadian amplitude, which is reflected in the performance curve in vigilance tasks ( Blatter et al 2006 ). Also, changes in the homeostatic process may play a role. During wakefulness, the accumulation of sleep pressure seems to be reduced in aging ( Murillo-Rodriguez et al 2004 ), which could leave older subjects more alert. There is also evidence that aging subjects recover faster from SD than young subjects in terms of physiological sleep ( Bonnet and Rosa 1987 ; Brendel et al 1990 ). This faster recovery in sleep state may also mean better restoration of cognitive performance ( Bonnet and Rosa 1987 ; Brendel et al 1990 ). However, more research is necessary to confirm these hypotheses.
The age effect found in previous studies could also be explained by methodological factors, such as inadequate control of the baseline conditions. Younger subjects are usually more chronically sleep deprived ( National Sleep Foundation 2002 ) due to several reasons, such as studying, career building or raising children. Chronic sleep restriction may cause long-term changes in brain functions that are not reversible during short adaptation and baseline periods in sleep laboratory studies. Even though subjects of certain studies were instructed to maintain a regular 8 h sleep schedule for 3–5 days, this may not be enough to erase the previous “sleep debt” ( Brendel et al 1990 ; Philip et al 2004 ; Adam et al 2006 ). Furthermore, in the long run, people tend to get used to experiencing sleepiness ( Van Dongen et al 2003a ) and thus may not even recognize being chronically sleep deprived. Perhaps aging people also have more experience that helps them to cope with the challenges posed by SD. Nevertheless, based on the available studies, it is impossible to distinguish the factors behind the age effect.
There are dissimilarities between genders in sleep structure measured with polysomnography (for a review, see Manber and Armitage 1999 ). Furthermore, women of all ages report more sleeping problems than men ( Leger et al 2000 ). Sex hormones affect sleep through several mechanisms, both genomic and nongenomic, including neurochemical and vascular mechanisms (for a review, see Dzaja et al 2005 ). This ensures instant and short-term effects as well as long-term ones.
It is possible that physiological responses to SD are not equal among men and women. During SD of 38 h, EEG showed more sleep activity in men than in women during waking rest and cognitive performance ( Corsi-Cabrera et al 2003 ). Presumably, therefore, one recovery night of nine hours would be enough to restore waking EEG activity in men, but not in women. Only a few studies have examined gender differences in cognitive performance during SD. In a vigilance task, performance was more impaired in men but returned to the baseline level in both men and women after recovery sleep ( Corsi-Cabrera et al 2003 ). In another study, women performed better than men in verbal and in visuo-constructive tasks during 35 h SD ( Binks et al 1999 ). No gender differences were observed in word fluency, maintenance or suppression of attention, auditory attention or cognitive flexibility. In that study, however, only one point of measurement was included, and so the difference in performance could be caused by SD or initial distinctions between the gender groups.
Few attempts have been made to evaluate the effect of sex hormones on coping with SD. It has been suggested that hormone therapy, which is widely used for women during their menopausal transition to help alleviate climacteric symptoms, attenuates physiological stress response ( Lindheim et al 1992 ). However, after 25 h of total SD, no difference was observed between hormone therapy users and nonusers in visual episodic memory, visuomotor performance, verbal attention and shared attention ( Alhola et al 2005 ). In addition, during 40 h of SD, hormone therapy did not produce any advantage in reaction time or vigilance tasks ( Karakorpi et al 2006 ).
The previous studies suggest that women cope with continuous wakefulness better than men. According to evolution, the demands of child nurturing and rearing in women would support this hypothesis ( Corsi-Cabrera et al 2003 ), but that certainly does not constitute a comprehensive explanation today. Gender differences during SD could be due to either physiological or social factors. There are differences in the brain structure and functioning of men and women ( Ragland et al 2000 ; Cowell et al 2007 ). These can be seen in cognitive performance in normal, non-deprived conditions: men typically have better spatial abilities and mental rotation, and higher visuo-constructive performance, whereas women perform better in visuomotor speed and some verbal functions, especially verbal fluency (for a review, see Kimura 1996 ). Men and women also exhibit behavioral and lifestyle differences, which are mainly due to socialization and gender roles ( Eagly and Wood 1999 ). Current literature, however, provides only minimal evidence of differential effects during SD, and does not resolve the issue of sexual dimorphism in coping with SD.
Interindividual differences
Several studies provide evidence that during total SD, performance becomes more variable as assessed from the within-subject point of view (eg, Smith et al 2002 ; Habeck et al 2004 ; Choo et al 2005 ). This is considered to reflect the wake-state instability caused by prolonged wakefulness. However, Doran et al (2001) were probably the first to also examine between-subjects variability, which they found to increase in PVT as wakefulness was extended to 88 hours. They suggested that some people are more vulnerable to the effects of sleep loss than others, which could probably explain the lack of significant results in some group comparisons. These differences between subjects could have arguably been caused by differences in sleep history, but the sleep patterns for the preceding week were controlled with sleep diaries, actigraph, and calls to the time-stamped voice recorder.
The interindividual variability has been further examined with a thorough protocol where a three night study (baseline, 36 h SD and recovery) was carried out three times ( Van Dongen et al 2004 ). Sleep history was manipulated by instructing subjects to stay in bed for either 6 or 12 h per night for one week before the study. The 12 h procedure was repeated and the order of the conditions was counterbalanced. The cognitive test selection included serial addition/subtraction task, digit symbol, critical tracking, word detection, repeated acquisition of response sequences, and PVT. The authors concluded that interindividual differences were systematic and independent from sleep history. The trait-like differential vulnerability to sleep loss has received support from an fMRI study attempting to reveal the neural basis for the interindividual differences ( Chuah et al 2006 ). They used a go/no-go task to measure response inhibition after 24 h of sleep deprivation. The results indicated that the subjects less vulnerable to SD had lower prefrontal cortex activation at the rested wakefulness than the more vulnerable subjects. During SD, activation increased temporarily in the prefrontal cortex and in some other areas only in the less vulnerable subjects. Since interindividual differences have also been found in other sleep-related variables, such as duration, timing, and quality of sleep, sleepiness, and circadian phase ( Van Dongen 1998 ; Van Dongen et al 2005 ), it is plausible that the tolerance to SD may also vary. Nevertheless, more studies are needed for further support.
Methodological issues and common biases
Although the adverse effects of SD on cognitive performance are quite well established, some studies have failed to detect any deterioration. Inadequate descriptions of study protocols or subject characteristics in some studies make it difficult to interpret the neutral results. However, it is likely that such results are due to methodological shortcomings, such as insensitive cognitive measures, failure to control the practice effect or other confounding factors, like individual sleep history or napping during the study. Also, if the task is carried out only once during the SD period, the results may be influenced by circadian rhythm.
Sleep deprivation studies are laborious and expensive to carry out, which may lead to compromises in the study design: for example, a small sample size can reduce the statistical power of the study, but a larger population may come at the expense of other methodological issues, such as a reduction in the cognitive test selection or in the number of nights spent in the sleep laboratory. Comparison of the results is also complicated because the length of sleep restriction varies and the studies are designed either within- or between-subjects.
Sleeping in unfamiliar surroundings may impair sleep quality. An adaptation night at the sleep laboratory is used to minimize this first night effect. However, in several studies, this has been neglected and the SD period has been preceded by a “normal” night at home (eg, Harrison and Horne 2000 ; Jennings et al 2003 ; Choo et al 2005 ). Although sleeping at home certainly reflects a subject’s reality more accurately, it does not allow for precise control and information of sleeping conditions. Adding a portable recording, such as an actigraph, provides objective information about eg, bedtime and resting periods. In some studies, the first night in the sleep laboratory has been the baseline (eg, Drummond et al 2000 ; Forest and Godbout 2000 ; De Gennaro et al 2001 ; Drummond et al 2001 ), whereas others have included one adaptation night (eg, Casagrande et al 1997 ; Alhola et al 2005 ). Yet, it may be questionable to use data from the second night as the baseline because sleep quality can be better than normal due to the rebound from the first night. Accordingly, only data from the third night should be accepted, which has been the case in a few studies ( Thomas et al 2000 ; Van Dongen et al 2003a ). This, however, makes the procedure very hard. Furthermore, study protocols can be improved by adding an ambulatory EEG recording to confirm the wakefulness of the subjects during the study.
In sleep studies, a common pitfall is recruitment methods. Enrolment via advertisements or from sleep clinics favors the selection of subjects with sleeping problems or concerns about their cognitive performance. Thus, strict exclusion criteria regarding physical or mental diseases or sleeping problems are essential. Further, sleeping habits should be controlled to make sure that the subjects are not initially sleep deprived. For this, use of a sleep diary for eg, 1–3 weeks before the experiment (eg, De Gennaro et al 2001 ; Habeck et al 2004 ; Alhola et al 2005 ) or an actigraph is applicable ( Harrison and Horne 1999 ; Thomas et al 2000 ).
The use of medication or stimulants, such as caffeine, alcohol or tobacco, is often prohibited before the experiment (eg, Thomas et al 2000 ; Van Dongen et al 2003a ; Habeck et al 2004 ; Alhola et al 2005 ; Choo et al 2005 ). In some studies, the subjects have been required to refrain from these substances only 24 h before the study ( Habeck et al 2004 ; Choo et al 2005 ), which may increase withdrawal symptoms and dropping out of the study. Thus a longer abstinence, eg, 1–2 weeks, is more appropriate ( Van Dongen et al 2003a ; Alhola et al 2005 ).
A variety of cognitive tests, from simple reaction time measures to complex decision-making tasks requiring creativity and reasoning, have been used to evaluate the effect of SD on cognition. The greatest problem in repeated cognitive testing is the practice effect, which easily conceals any adverse effects of SD. Therefore, careful control over learning is essential. Cognitive processes are also intertwined in several ways, which makes it difficult to specify exactly which cognitive functions are utilized in certain performances. Because attention is involved in performing any cognitive task, a decrease in other cognitive domains during SD may be mediated through impaired attention. In complex tasks, however, applying previous knowledge and use of strategies or creativity may be more essential. Some studies have concentrated on neural correlates of cognitive functioning during continuous wakefulness. Both fMRI ( Portas et al 1998 ; Drummond et al 2000 ; Drummond et al 2001 ; Chee and Choo 2004 ; Habeck et al 2004 ; Choo et al 2005 ) and PET have been used ( Thomas et al 2000 ). Although these trials yield interesting information about brain functioning, the use of imaging techniques limits the selection of cognitive tests that could be carried out at the same time.
Dorrian et al (2005) have compiled a list of criteria for neurocognitive tests that would be suitable for investigating sleep deprivation effects. The criteria include psychometric quality, ie, reliability and validity, but the tests should also reflect a fundamental aspect of waking neurocognitive functions and it should be possible to interpret them in a meaningful way. The tasks should be repeatable, independent of aptitude, and they should be short with a high signal load. These criteria are not met in some studies. Dorrian et al (2005) also argued that vigilance is the underlying factor through which the sleep deprivation effects are mediated in all other tasks. However, although attention is needed to perform any task to some extent, the hypothesis that sleep deprivation can have an independent effect on other cognitive functions such as memory cannot be ruled out. Nevertheless, when measuring other cognitive functions, the characteristics of the task should be considered carefully and, eg, for repeated measures of memory, parallel test versions should be used.
The negative effect of both acute total and chronic partial SD on attention and working memory is supported by existing literature. Total SD impairs a range of other cognitive functions as well. In partial SD, a more thorough evaluation of higher cognitive functions is needed. Furthermore, the effects of SD have not been thoroughly compared among some essential subpopulations.
Aging influences a person’s ability to cope with SD. Although in general the cognitive performance of aging people is often poorer than that of younger individuals, during SD performance in older subjects seems to deteriorate less. Based on the scarce evidence, it seems that in terms of cognitive performance, women may endure prolonged wakefulness better than men, whereas physiologically they recover slower. Tolerating SD can also depend on individual traits. However, mechanisms inducing differences between the young and aging and between men and women or different individuals are mostly unclear. Several reasons such as physiological mechanisms as well as social or environmental factors may be involved. In conclusion, there is great variation in SD studies in terms of both subject selections and methods, and this makes it difficult to compare the different studies. In the future, methodological issues should be considered more thoroughly.
- Achermann P. The two-process model of sleep regulation revisited. Aviat Space Environ Med. 2004;75:A37–43. [ PubMed ] [ Google Scholar ]
- Adam M, Retey JV, Khatami R, et al. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep. 2006;29:55–7. doi: 10.1093/sleep/29.1.55. [ DOI ] [ PubMed ] [ Google Scholar ]
- Akerstedt T, Folkard S. Validation of the S and C components of the three-process model of alertness regulation. Sleep. 1995;18:1–6. doi: 10.1093/sleep/18.1.1. [ DOI ] [ PubMed ] [ Google Scholar ]
- lhola P, Tallus M, Kylmälä M, et al. Sleep deprivation, cognitive performance, and hormone therapy in postmenopausal women. Menopause. 2005;12:149–55. doi: 10.1097/00042192-200512020-00008. [ DOI ] [ PubMed ] [ Google Scholar ]
- Armitage R, Smith C, Thompson S, et al. Sex differences in slow-wave activity in response to sleep deprivation. Sleep Research Online. 2001;4(1):33–41. [ Google Scholar ]
- Babkoff H, Zukerman G, Fostick L, et al. Effect of the diurnal rhythm and 24 h of sleep deprivation on dichotic temporal order judgment. J Sleep Res. 2005;14:7–15. doi: 10.1111/j.1365-2869.2004.00423.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Baddeley AD, Hitch GJ. Working memory . Academic Press; 1974. pp. 47–89. [ Google Scholar ]
- Baddeley A. The episodic buffer: A new component of working memory? Trends Cogn Sci. 2000;4:417–23. doi: 10.1016/s1364-6613(00)01538-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- Baddeley A, Cocchini G, Della Sala S, et al. Working memory and vigilance: Evidence from normal aging and alzheimer’s disease. Brain Cogn. 1999;41:87–108. doi: 10.1006/brcg.1999.1097. [ DOI ] [ PubMed ] [ Google Scholar ]
- Baranski JV, Pigeau RA. Self-monitoring cognitive performance during sleep deprivation: Effects of modafinil, d-amphetamine and placebo. J Sleep Res. 1997;6:84–91. doi: 10.1111/j.1365-2869.1997.00032.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Baranski JV, Pigeau RA, Angus RG. On the ability to self-monitor cognitive performance during sleep deprivation: A calibration study. J Sleep Res. 1994;3:36–44. doi: 10.1111/j.1365-2869.1994.tb00102.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Binks PG, Waters WF, Hurry M. Short-term total sleep deprivations does not selectively impair higher cortical functioning. Sleep. 1999;22:328–34. doi: 10.1093/sleep/22.3.328. [ DOI ] [ PubMed ] [ Google Scholar ]
- Blagrove M, Alexander C, Horne JA. The effects of chronic sleep reduction on the performance of cognitive tasks sensitive to sleep deprivation. App Cogn Psych. 1995;9:21–40. [ Google Scholar ]
- Blatter K, Graw P, Munch M, et al. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res. 2006;168:312–7. doi: 10.1016/j.bbr.2005.11.018. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bocca ML, Denise P. Total sleep deprivation effect on disengagement of spatial attention as assessed by saccadic eye movements. Clin Neurophysiol. 2006;117:894–9. doi: 10.1016/j.clinph.2006.01.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bonnet MH, Rosa RR. Sleep and performance in young adults and older normals and insomniacs during acute sleep loss and recovery. Biol Psychol. 1987;25:153–72. doi: 10.1016/0301-0511(87)90035-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- Brendel DH, Reynolds CF, 3rd, Jennings JR, et al. Sleep stage physiology, mood, and vigilance responses to total sleep deprivation in healthy 80-year-olds and 20-year-olds. Psychophysiology. 1990;27:677–85. doi: 10.1111/j.1469-8986.1990.tb03193.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Carskadon MA, Dement WC. Normal human sleep: An overview. Philadelphia: Elsevier Saunders; 2005. pp. 13–23. [ Google Scholar ]
- Casagrande M, Violani C, Curcio G, et al. Assessing vigilance through a brief pencil and paper letter cancellation task (LCT): Effects of one night of sleep deprivation and of the time of day. Ergonomics. 1997;40:613–30. doi: 10.1080/001401397187919. [ DOI ] [ PubMed ] [ Google Scholar ]
- Casement MD, Broussard JL, Mullington JM, et al. The contribution of sleep to improvements in working memory scanning speed: A study of prolonged sleep restriction. Biol Psychol. 2006;72:208–12. doi: 10.1016/j.biopsycho.2005.11.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chee MW, Chuah LY, Venkatraman V, et al. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of fronto-parietal activation with performance. Neuroimage. 2006;31:419–28. doi: 10.1016/j.neuroimage.2005.12.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- Choo WC, Lee WW, Venkatraman V, et al. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage. 2005;25:579–87. doi: 10.1016/j.neuroimage.2004.11.029. [ DOI ] [ PubMed ] [ Google Scholar ]
- Chuah YM, Venkatraman V, Dinges DF, et al. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26:7156–62. doi: 10.1523/JNEUROSCI.0906-06.2006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Corsi-Cabrera M, Sanchez AI, del-Rio-Portilla Y, et al. Effect of 38 h of total sleep deprivation on the waking EEG in women: Sex differences. Int J Psychophysiol. 2003;50:213–24. doi: 10.1016/s0167-8760(03)00168-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- Coull JT, Jones MEP, Egan TD, et al. Attentional effects of nor-adrenaline vary with arousal level: Selective activation of thalamic pulvinar in humans. NeuroImage. 2004/5;22:315–22. doi: 10.1016/j.neuroimage.2003.12.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- Cowell PE, Sluming VA, Wilkinson ID, et al. Effects of sex and age on regional prefrontal brain volume in two human cohorts. Eur J Neurosci. 2007;25:307–18. doi: 10.1111/j.1460-9568.2006.05281.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- De Gennaro L, Ferrara M, Curcio G, et al. Visual search performance across 40 h of continuous wakefulness: Measures of speed and accuracy and relation with oculomotor performance. Physiol Behav. 2001;74:197–204. doi: 10.1016/s0031-9384(01)00551-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–9. doi: 10.1172/JCI117184. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [ PubMed ] [ Google Scholar ]
- Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Instr Comp. 1985;17:652–5. [ Google Scholar ]
- Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: Evidence of state instability. Arch Ital Biol. 2001;139:253–67. [ PubMed ] [ Google Scholar ]
- Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. New York: Marcel Dekker; 2005. pp. 39–70. [ Google Scholar ]
- Drummond SP, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25:S68–73. doi: 10.1016/S0893-133X(01)00325-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- Drummond SP, Brown GG, Gillin JC, et al. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [ DOI ] [ PubMed ] [ Google Scholar ]
- Drummond SP, Brown GG, Salamat JS, et al. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep. 2004;27:445–51. [ PubMed ] [ Google Scholar ]
- Drummond SP, Brown GG, Stricker JL, et al. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10:3745–8. doi: 10.1097/00001756-199912160-00004. [ DOI ] [ PubMed ] [ Google Scholar ]
- Drummond SP, Gillin JC, Brown GG. Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res. 2001;10:85–92. doi: 10.1046/j.1365-2869.2001.00245.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006;15:261–5. doi: 10.1111/j.1365-2869.2006.00535.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dzaja A, Arber S, Hislop J, et al. Women’s sleep in health and disease. J Psychiatr Res. 2005;39:55–76. doi: 10.1016/j.jpsychires.2004.05.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- Eagly AH, Wood W. The Origins of Sex Differences in Human Behavior: Evolved Dispositions Versus Social Roles. Am Psychol. 1999;54:408–23. [ Google Scholar ]
- Forest G, Godbout R. Effects of sleep deprivation on performance and EEG spectral analysis in young adults. Brain Cogn. 2000;43:195–200. [ PubMed ] [ Google Scholar ]
- Frey DJ, Badia P, Wright KP., Jr Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004;13:305–15. doi: 10.1111/j.1365-2869.2004.00429.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gaudreau H, Carrier J, Tchiteya B. Age and individual determinants of sleep loss effects. New York: Marcel Dekker; 2005. pp. 441–479. [ Google Scholar ]
- Gosselin A, De Koninck J, Campbell KB. Total sleep deprivation and novelty processing: Implications for frontal lobe functioning. Clin Neurophysiol. 2005;116:211–22. doi: 10.1016/j.clinph.2004.07.033. [ DOI ] [ PubMed ] [ Google Scholar ]
- Graw P, Krauchi K, Knoblauch V, et al. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav. 2004;80:695–701. doi: 10.1016/j.physbeh.2003.12.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- Habeck C, Rakitin BC, Moeller J, et al. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Brain Res Cogn Brain Res. 2004;18:306–21. doi: 10.1016/j.cogbrainres.2003.10.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- Harrison Y, Espelid E. Loss of negative priming following sleep deprivation. Q J Exp Psychol A. 2004;57:437–46. doi: 10.1080/02724980343000224. [ DOI ] [ PubMed ] [ Google Scholar ]
- Harrison Y, Horne JA. Sleep loss and temporal memory. Q J Exp Psychol A. 2000;53:271–9. doi: 10.1080/713755870. [ DOI ] [ PubMed ] [ Google Scholar ]
- Harrison Y, Horne JA. One night of sleep loss impairs innovative thinking and flexible decision making. Organ Behav Hum Decis Process. 1999;78:128–45. doi: 10.1006/obhd.1999.2827. [ DOI ] [ PubMed ] [ Google Scholar ]
- Harrison Y, Horne JA. Sleep loss impairs short and novel language tasks having a prefrontal focus. J Sleep Res. 1998;7:95–100. doi: 10.1046/j.1365-2869.1998.00104.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults–a model for healthy aging? Sleep. 2000;23:1067–73. [ PubMed ] [ Google Scholar ]
- Heuer H, Klein W. One night of total sleep deprivation impairs implicit learning in the serial reaction task, but not the behavioral expression of knowledge. Neuropsychology. 2003;17:507–16. doi: 10.1037/0894-4105.17.3.507. [ DOI ] [ PubMed ] [ Google Scholar ]
- Heuer H, Kleinsorge T, Klein W, et al. Total sleep deprivation increases the costs of shifting between simple cognitive tasks. Acta Psychol (Amst) 2004;117:29–64. doi: 10.1016/j.actpsy.2004.04.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- Heuer H, Kohlisch O, Klein W. The effects of total sleep deprivation on the generation of random sequences of key-presses, numbers and nouns. Q J Exp Psychol A. 2005;58:275–307. doi: 10.1080/02724980343000855. [ DOI ] [ PubMed ] [ Google Scholar ]
- Heuer H, Spijkers W, Kiesswetter E, et al. Effects of sleep loss, time of day, and extended mental work on implicit and explicit learning of sequences. J Exp Psychol Appl. 1998;4:139–62. doi: 10.1037//1076-898x.4.2.139. [ DOI ] [ PubMed ] [ Google Scholar ]
- Horne JA. Human sleep, sleep loss and behaviour. implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993;162:413–9. doi: 10.1192/bjp.162.3.413. [ DOI ] [ PubMed ] [ Google Scholar ]
- Horne JA, Pettitt AN. High incentive effects on vigilance performance during 72 hours of total sleep deprivation. Acta Psychol (Amst) 1985;58:123–39. doi: 10.1016/0001-6918(85)90003-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hwang DY, Golby AJ. The brain basis for episodic memory: Insights from functional MRI, intracranial EEG, and patients with epilepsy. Epilepsy Behav. 2006;8:115–26. doi: 10.1016/j.yebeh.2005.09.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- Jennings JR, Monk TH, van der Molen MW. Sleep deprivation influences some but not all processes of supervisory attention. Psychol Sci. 2003;14:473–9. doi: 10.1111/1467-9280.02456. [ DOI ] [ PubMed ] [ Google Scholar ]
- Johnsen BH, Laberg JC, Eid J, et al. Dichotic listening and sleep deprivation: Vigilance effects. Scand J Psychol. 2002;43:413–17. doi: 10.1111/1467-9450.00309. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kalleinen N, Polo O, Himanen SL, et al. Sleep deprivation and hormone therapy in postmenopausal women. Sleep Med. 2006;7:436–47. doi: 10.1016/j.sleep.2006.02.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- Karakorpi M, Alhola P, Urrila AS, et al. Hormone treatment gives no benefit against cognitive changes caused by acute sleep deprivation in postmenopausal women. Neuropsychopharmacology. 2006;31:2079–88. doi: 10.1038/sj.npp.1301056. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kendall AP, Kautz MA, Russo MB, et al. Effects of sleep deprivation on lateral visual attention. Int J Neurosci. 2006;116:1125–38. doi: 10.1080/00207450500513922. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kilduff TS, Kushida CA, Terao A. Recovery from sleep deprivation. New York: Marcel Dekker; 2005. pp. 485–502. [ Google Scholar ]
- Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kim DJ, Lee HP, Kim MS, et al. The effect of total sleep deprivation on cognitive functions in normal adult male subjects. Int J Neurosci. 2001;109:127–37. doi: 10.3109/00207450108986529. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kimura D. Sex, sexual orientation and sex hormones influence human cognitive function. Curr Opin Neurobiol. 1996;6:259–63. doi: 10.1016/s0959-4388(96)80081-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kjellberg A. Sleep deprivation and some aspects of performance. II. lapses and other attentional effects. Waking Sleeping. 1977;1:145–8. [ Google Scholar ]
- Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kronholm E, Harma M, Hublin C, et al. Self-reported sleep duration in finnish general population. J Sleep Res. 2006;15:276–90. doi: 10.1111/j.1365-2869.2006.00543.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lac G, Chamoux A. Elevated salivary cortisol levels as a result of sleep deprivation in a shift worker. Occup Med (Lond) 2003;53:143–5. doi: 10.1093/occmed/kqg028. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lee HJ, Kim L, Suh KY. Cognitive deterioration and changes of P300 during total sleep deprivation. Psychiatry Clin Neurosci. 2003;57:490–6. doi: 10.1046/j.1440-1819.2003.01153.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Leger D, Guilleminault C, Dreyfus JP, et al. Prevalence of insomnia in a survey of 12,778 adults in france. J Sleep Res. 2000;9:35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Linde L, Bergstrom M. The effect of one night without sleep on problem-solving and immediate recall. Psychol Res. 1992;54:127–36. doi: 10.1007/BF00937141. [ DOI ] [ PubMed ] [ Google Scholar ]
- Linde L, Edland A, Bergström M. Auditory attention and multi-attribute decision-making during a 33 h sleep-deprivation period: Mean performance and between-subject dispersions. Ergonomics. 1999;42:696–713. doi: 10.1080/001401399185397. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lindheim SR, Legro RS, Bernstein L, et al. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol. 1992;167:1831–6. doi: 10.1016/0002-9378(92)91783-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- Manber R, Armitage R. Sex, steroids, and sleep: A review. Sleep. 1999;22:540–55. [ PubMed ] [ Google Scholar ]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [ DOI ] [ PubMed ] [ Google Scholar ]
- McCarthy ME, Waters WF. Decreased attentional responsivity during sleep deprivation: Orienting response latency, amplitude, and habituation. Sleep. 1997;20:115–23. doi: 10.1093/sleep/20.2.115. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mikulincer M, Babkoff H, Caspy T, et al. The effects of 72 hours of sleep loss on psychological variables. Br J Psychol. 1989;80 (Pt 2):145–62. doi: 10.1111/j.2044-8295.1989.tb02309.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Monk TH, Carrier J. Speed of mental processing in the middle of the night. Sleep. 1997;20:399–401. doi: 10.1093/sleep/20.6.399. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mu Q, Nahas Z, Johnson KA, et al. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005;28:55–67. doi: 10.1093/sleep/28.1.55. [ DOI ] [ PubMed ] [ Google Scholar ]
- Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, et al. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004;123:361–70. doi: 10.1016/j.neuroscience.2003.09.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: Shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- National Sleep Foundation. [Accessed 8 March 2007];“Sleep in America” poll. 2007 :1–52. URL: http://www.sleepfoundation.org .
- National Sleep Foundation. [Accessed 6 September 2006];“Sleep in America” poll. 2002 :1–44. URL: http://www.sleepfoundation.org .
- Nilsson JP, Soderstrom M, Karlsson AU, et al. Less effective executive functioning after one night’s sleep deprivation. J Sleep Res. 2005;14:1–6. doi: 10.1111/j.1365-2869.2005.00442.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ogawa Y, Kanbayashi T, Saito Y, et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: A study with microneurographic technique. Sleep. 2003;26:986–9. doi: 10.1093/sleep/26.8.986. [ DOI ] [ PubMed ] [ Google Scholar ]
- Otmani S, Pebayle T, Roge J, et al. Effect of driving duration and partial sleep deprivation on subsequent alertness and performance of car drivers. Physiol Behav. 2005;84:715–24. doi: 10.1016/j.physbeh.2005.02.021. [ DOI ] [ PubMed ] [ Google Scholar ]
- Philibert I. Sleep loss and performance in residents and nonphysicians; a meta-analytic examination. Sleep. 2005;28:1393–402. doi: 10.1093/sleep/28.11.1392. [ DOI ] [ PubMed ] [ Google Scholar ]
- Philip P, Taillard J, Sagaspe P, et al. Age, performance and sleep deprivation. J Sleep Res. 2004;13:105–10. doi: 10.1111/j.1365-2869.2004.00399.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: A meta-analysis. Sleep. 1996;19:318–26. doi: 10.1093/sleep/19.4.318. [ DOI ] [ PubMed ] [ Google Scholar ]
- Portas CM, Rees G, Howseman AM, et al. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18:8979–89. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Priest B, Brichard C, Aubert G, et al. Microsleep during a simplified maintenance of wakefulness test. A validation study of the OSLER test. Am J Respir Crit Care Med. 2001;163:1619–25. doi: 10.1164/ajrccm.163.7.2007028. [ DOI ] [ PubMed ] [ Google Scholar ]
- Quigley N, Green JF, Morgan D, et al. The effect of sleep deprivation on memory and psychomotor function in healthy volunteers. Hum Psychopharmacol. 2000;15:171–7. doi: 10.1002/(SICI)1099-1077(200004)15:3<171::AID-HUP155>3.0.CO;2-D. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ragland JD, Coleman AR, Gur RC, et al. Sex differences in brain-behavior relationships between verbal episodic memory and resting regional cerebral blood flow. Neuropsychologia. 2000;38:451–61. doi: 10.1016/s0028-3932(99)00086-x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Raidy DJ, Scharff LF. Effects of sleep deprivation on auditory and visual memory tasks. Percept Mot Skills. 2005;101:451–67. doi: 10.2466/pms.101.2.451-467. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rinkenauer G, Osman A, Ulrich R, et al. On the locus of speed-accuracy trade-off in reaction time: Inferences from the lateralized readiness potential. J Exp Psychol Gen. 2004;133:261–82. doi: 10.1037/0096-3445.133.2.261. [ DOI ] [ PubMed ] [ Google Scholar ]
- Russo MB, Kendall AP, Johnson DE, et al. Visual perception, psychomotor performance, and complex motor performance during an overnight air refueling simulated flight. Aviat Space Environ Med. 2005;76:C92–103. [ PubMed ] [ Google Scholar ]
- Sagaspe P, Sanchez-Ortuno M, Charles A, et al. Effects of sleep deprivation on color-word, emotional, and specific stroop interference and on self-reported anxiety. Brain Cogn. 2006;60:76–87. doi: 10.1016/j.bandc.2005.10.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- Samkoff JS, Jacques CH. A review of studies concerning effects of sleep deprivation and fatigue on residents’ performance. Acad Med. 1991;66:687–93. doi: 10.1097/00001888-199111000-00013. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: The neurobiology of attentional effort. Brain Res Brain Res Rev. 2006;51:145–60. doi: 10.1016/j.brainresrev.2005.11.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci. 2000;12:103–13. doi: 10.1176/jnp.12.1.103. [ DOI ] [ PubMed ] [ Google Scholar ]
- Shneerson JM. Handbook of sleep medicine. Cambridge: Blackwell Science; 2000. [ Google Scholar ]
- Smith ME, McEvoy LK, Gevins A. The impact of moderate sleep loss on neurophysiologic signals during working-memory task performance. Sleep. 2002;25:784–94. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smulders FT, Kenemans JL, Jonkman LM, et al. The effects of sleep loss on task performance and the electroencephalogram in young and elderly subjects. Biol Psychol. 1997;45:217–39. doi: 10.1016/s0301-0511(96)05229-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- Spiegel K, Knutson K, Leproult R, et al. Sleep loss: A novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [ DOI ] [ PubMed ] [ Google Scholar ]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- Stenuit P, Kerkhofs M. Age modulates the effects of sleep restriction in women. Sleep. 2005;28:1284–8. doi: 10.1093/sleep/28.10.1283. [ DOI ] [ PubMed ] [ Google Scholar ]
- Steyvers FJ. The influence of sleep deprivation and knowledge of results on perceptual encoding. Acta Psychol (Amst) 1987;66:173–87. doi: 10.1016/0001-6918(87)90032-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- Steyvers FJ, Gaillard AW. The effects of sleep deprivation and incentives on human performance. Psychol Res. 1993;55:64–70. doi: 10.1007/BF00419894. [ DOI ] [ PubMed ] [ Google Scholar ]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [ DOI ] [ PubMed ] [ Google Scholar ]
- Strangman G, Thompson JH, Strauss MM, et al. Functional brain imaging of a complex navigation task following one night of total sleep deprivation: A preliminary study. J Sleep Res. 2005;14:369–75. doi: 10.1111/j.1365-2869.2005.00488.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Swann CE, Yelland GW, Redman JR, et al. Chronic partial sleep loss increases the facilitatory role of a masked prime in a word recognition task. J Sleep Res. 2006;15:23–9. doi: 10.1111/j.1365-2869.2006.00495.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Taillard J, Moore N, Claustrat B, et al. Nocturnal sustained attention during sleep deprivation can be predicted by specific periods of subjective daytime alertness in normal young humans. J Sleep Res. 2006;15:41–5. doi: 10.1111/j.1365-2869.2006.00505.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Thorne DR, Thomas ML, Sing HC, et al. Driving-simulator accident rates before, during and after one week of restricted nightly sleep. Sleep. 1999;21:S135. [ Google Scholar ]
- Tomporowski PD, Tinsley VF. Effects of memory demand and motivation on sustained attention in young and older adults. Am J Psychol. 1996;109:187–204. [ PubMed ] [ Google Scholar ]
- Tsai LL, Young HY, Hsieh S, et al. Impairment of error monitoring following sleep deprivation. Sleep. 2005;28:707–13. doi: 10.1093/sleep/28.6.707. [ DOI ] [ PubMed ] [ Google Scholar ]
- Van Dongen HP, Baynard MD, Maislin G, et al. Systematic interin-dividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [ PubMed ] [ Google Scholar ]
- Van Dongen HP, Maislin G, Mullington JM, et al. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003a;26:117–26. doi: 10.1093/sleep/26.2.117. [ DOI ] [ PubMed ] [ Google Scholar ]
- Van Dongen HP, Rogers NL, Dinges DF. Sleep debt: Theoretical and empirical issues. Sleep Biol Rhythms. 2003b;1:5–13. [ Google Scholar ]
- Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–96. doi: 10.1093/sleep/28.4.479. [ DOI ] [ PubMed ] [ Google Scholar ]
- Van Dongen HPA. Inter- and intra-individual differences in circadian phase 1998 [ Google Scholar ]
- Versace F, Cavallero C, De Min Tona G, et al. Effects of sleep reduction on spatial attention. Biol Psychol. 2006;71:248–55. doi: 10.1016/j.biopsycho.2005.04.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wilkinson RT. Response-stimulus interval in choice serial reaction time: Interaction with sleep deprivation, choice, and practice. Q J Exp Psychol A. 1990;42:401–23. doi: 10.1080/14640749008401228. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wimmer F, Hoffmann RF, Bonato RA, et al. The effects of sleep deprivation on divergent thinking and attention processes. J Sleep Res. 1992;1:223–30. doi: 10.1111/j.1365-2869.1992.tb00043.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wright KP, Jr, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 1999;103:185–94. doi: 10.1016/s0166-4328(99)00042-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wu JC, Gillin JC, Buchsbaum MS, et al. The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991;14:155–62. [ PubMed ] [ Google Scholar ]
- Zils E, Sprenger A, Heide W, et al. Differential effects of sleep deprivation on saccadic eye movements. Sleep. 2005;28:1109–15. doi: 10.1093/sleep/28.9.1109. [ DOI ] [ PubMed ] [ Google Scholar ]
- PDF (199.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 16 December 2024
Sleep on it: exploring the psychology of sleep amidst contemporary challenges
- Kristina Denisova ORCID: orcid.org/0000-0002-0104-019X 1 ,
- Yuki Motomura 2 &
- Chen Song ORCID: orcid.org/0000-0002-5418-5747 3
Scientific Reports volume 14 , Article number: 30501 ( 2024 ) Cite this article
1755 Accesses
1 Altmetric
Metrics details
- Human behaviour
- Risk factors
Throughout history, poets, scholars, and scientists have acknowledged the profound link between sleep and psychological well-being. The wisdom of “sleep on it”, ingrained in both Western and Eastern traditions, highlights the crucial role sleep plays in restoring and enhancing cognitive functions. In today’s fast-paced, highly-interconnected, technology-driven world, where cognitive demands are ever-growing, quality sleep has become both more vital and more elusive. This collection delves into the evolving role of sleep in maintaining psychological well-being amidst contemporary challenges. It brings together a diverse array of behavioral and brain imaging studies from researchers across the globe, focusing on three key areas: the beneficial effects of sleep on learning and education, the detrimental consequences of sleep disruption on mental health, and the rising prevalence of sleep disruption in vulnerable populations. These studies offer compelling insights, revealing, for instance, how sleep consolidates conceptual networks of knowledge, how sleep disruption can signal suicidal tendencies a month before suicide attempts, and how heatwaves negatively affect infant sleep. This body of work not only underscores the cognitive benefits of sleep but also illuminates how contemporary challenges—such as climate change, poverty, and shift work—undermine sleep health. It calls for targeted interventions to improve sleep health and psychological well-being in response to these contemporary challenges, urging scholars and policymakers to prioritize sleep health as a foundational element in building a healthier, more resilient society.
Introduction
Historically, sleep has been regarded as a cornerstone of psychological well-being. As early as the fifth century BC, Sophocles described sleep as “a stranger to anguish” with the “power to heal” 1 . In the first millennium AD, Ovid referred to sleep as “the spirit’s comforter, router of care” 2 . Moving to the seventeenth century, William Shakespeare recognized sleep as the “balm of hurt minds” 3 and attributed special value to dreams 4 . In the nineteenth and twentieth centuries, researchers, notably Marie de Manacéïne, proposed that sleep and dreaming are crucial sources of intellectual inspiration, emphasizing the notion of “sleep on it” 5 . This notion is deeply embedded in the collective wisdom of various cultures, as reflected in proverbs such as “we must always sleep over an important resolution” and “morning is the time to make up one’s mind” 5 . It highlights the crucial role sleep plays in restoring and enhancing a wide range of cognitive functions 6 , such as decision-making, problem-solving, emotion regulation, memory, learning, all of which are fundamental to psychological well-being 6 , 7 , 8 , 9 , 10 .
As we advance into a fast-paced technological age, with growing socioeconomic and environmental challenges, maintaining quality sleep becomes both increasingly important and increasingly difficult. Navigating the demands of this highly interconnected world requires optimal cognitive performance and new cognitive strategies, which can only be sustained through quality sleep. However, contemporary stress often leads to reduced sleep time, creating a paradox where we need sleep more than ever but have less of it. This dilemma raises new research questions along two lines. First, what role does sleep play in supporting new cognitive and learning strategies needed for navigating contemporary society? Second, how do the challenges of contemporary society affect sleep and mental health?
Articles in this collection address these key questions, providing new evidence on sleep’s role in maintaining psychological well-being, while also shedding light on the adverse impacts that contemporary society imposes on sleep. Although our physiological need for sleep remains unchanged, the psychological necessity for high-quality sleep has intensified with contemporary challenges. This collection focuses on the link between sleep and psychological well-being, distinct from the important but separate theme of sleep physiology. Our call for papers was answered by researchers across the globe, whose work addresses the psychology of sleep from various angles, including learning, education and mental health. They also examine sensitive and timely issues, such as the impacts of poverty and climate change on sleep, providing valuable insights for public health bodies and policy makers. We highlight some of these findings below (see Fig. 1 for a word cloud summary of the topics covered).

A word cloud summary based on titles and abstracts of articles in this collection.
The beneficial effects of sleep on learning and education
Sleep is essential for cognitive development; it helps the brain integrate new and existing knowledge, making learning long-lasting rather than transient 8 , 9 , 10 . Building on previous research, articles in this collection provide new insights into the role sleep plays in learning and memory, with implications for education.
Using an innovative teletransportation paradigm, Feld and colleagues 11 discovered that sleep enhances memorization of conceptual links between items, facilitating the construction of internal knowledge networks. Vidal and colleagues 12 showed that memory reactivation during sleep can improve the consolidation of complex classroom knowledge, demonstrating the educational benefits of memory reactivation techniques. Combining dream reports with an emotional picture task, Zhang and colleagues 13 observed an active role for dreaming in emotional memory processing. Gonzales and colleagues 14 found that early chronotypes exhibit greater prefrontal brain activity during a visuospatial working memory task, suggesting that early rising may protect brain function and cognitive reserve.
These findings open up new avenues for future research. In this highly interconnected world, memorizing conceptual links between knowledge is becoming increasingly important. Future research could investigate how the construction of knowledge networks differs between neurotypical children and those experiencing sleep disruption, such as autistic children, while controlling for IQ 15 , 16 , 17 . If autistic children perform better, this could point to compensatory (or alternative) mechanisms that enable the construction of knowledge networks despite sleep challenges. Such mechanisms might involve local sleep during wakefulness, where specific brain regions enter a sleep-like state while the individual remains awake and active 18 , 19 , 20 . Understanding these mechanisms could help inform educational strategies that algin with sleep patterns to optimize cognitive benefits.
The detrimental consequences of sleep disruption on mental health
Sleep disruption is a common comorbidity in many neuropsychiatric conditions, including autism, anxiety, and depression, each exhibiting distinct alterations in polysomnography profiles 21 . Previous research has linked sleep difficulties to internalizing and externalizing symptoms in adolescents 22 and young adults. Articles in this collection provide further insights into how external (environmental) factors, such as the COVID-19 pandemic, and internal factors, such as suicidal ideation, influence sleep patterns in the youth population.
Rolling and colleagues 23 investigated sleep patterns in adolescents who had attempted suicide, discovering that, in the month preceding suicide attempts, these individuals experienced shorter total sleep time and longer sleep latency on school days. Bauducco and colleagues 24 found that during the COVID-19 pandemic, declining mental health in adolescents was closely tied to deteriorating sleep patterns. Reynolds and colleagues 25 compared young adults with and without shift jobs, revealing that sleep disorders, along with elevated levels of anxiety and depression, were more prevalent among young shift workers, regardless of their shift schedules.
An open question in the field is the direction of causality between sleep disruption and mental health disturbances. Recent evidence suggests that sleep disruption may precede mental health symptoms, with the early onset of low-quality sleep in infancy linked to atypical socio-emotional developmental trajectories 26 . However, the shared genetic and environmental factors underying sleep patterns 8 and neuropsychiatric conditions 27 complicate causal inference. Access to large-scale genetic datasets could help uncover whether sleep disruption instigates mental health disturbances, or vice versa.
The escalation of sleep disruption amidst contemporary challenges
Understanding the effects of poverty and lower socioeconomic status on sleep is crucial, but research in this area, particularly from developing countries, is limited. Moreover, the impacts of climate change and rising urban temperatures on sleep remain largely unexplored. Articles in this collection explicitly address these knowledge gaps.
Berger and colleagues 28 discovered that infant sleep was significantly disrupted during a summer heatwave in the UK, with reduced total sleep, more fragmented sleep, and more frequent parental visits to the crib, compared to non-heatwave nights. Correia and colleagues 29 examined how socioeconomic status and stress level affected sleep in South African adults, finding that participants with safety-related fears reported poorer sleep quality, despite having relatively long sleep durations (over nine hours). This pattern contrasts with observations among African Americans in the U.S, highlighting the importance of identifying unique sleep disruption patterns in diverse populations.
Since sleep disruption can foreshadow mental health disturbances, understanding the effects of environmental and socioeconomic stressors on sleep, particularly in vulnerable populations, is essential for preventing long-term psychological issues. Findings in this collection suggest that interventions aimed at improving sleep quality should be tailored to specific populations and their unique circumstances. For instance, developing affordable and sustainable cooling solutions in urban areas could help low-income families mitigate the impacts of climate change on sleep. Similarly, community-based initiatives designed to enhance security and reduce stress might alleviate safety-related sleep disruptions in high-crime areas. Building on these findings, future research could incorporate genetic data to address how the interplay between genetic predispositions and environmental or socioeconomic factors shapes sleep patterns and psychological well-being 30 .
Sleep is far more than a passive state of rest; it is an active process crucial to cognitive function and mental health. This collection highlights the beneficial effects of sleep on learning and education, as well as the adverse impacts of sleep disruption on mental health. It emphasizes that high-quality sleep is not merely a physiological necessity but a psychological one, essential for navigating the complexities of contemporary society. As socioeconomic and environmental pressures rise, ensuring equitable access to sleep becomes a pressing public health priority. By examining the psychology of sleep in the context of contemporary challenges, the field can move towards a more comprehensive and inclusive understanding of sleep’s importance for psychological well-being, ultimately leading to better health outcomes and quality of life across diverse populations. This collection thus serves as both a call to action and a roadmap for future research, encouraging scholars and policymakers alike to prioritize sleep health for a healthier, more resilient society.
Sophocles. In Greek Drama (ed Moses Hadas) 172 (Bantam Books, 1982).
Ovid. In Metamorphoses: Book XI . 278 (Indiana University Press, 1955).
Shakespeare. In W. Macbeth . A Norton Critical edn, 27 (Norton, 2004).
Shakespeare. In W. Hamlet . A Norton Critical Edition; 2nd edn, (Norton, 1992).
Manacéïne, M. Sleep, its physiology, pathology, hygiene, and psychology Vol. 325 (The Walter Scott Press, 1897).
Book Google Scholar
Kleitman, N. Sleep and Wakefulness (The University of Chicago Press, 1939).
Google Scholar
Kandel, E. The new science of mind and the future of knowledge. Neuron 80 , 546–560. https://doi.org/10.1016/j.neuron.2013.10.039 (2013).
Article CAS PubMed Google Scholar
Rasch, B. & Born, J. About sleep’s role in memory. Physiol. Rev. 93 , 681–766. https://doi.org/10.1152/physrev.00032.2012 (2013).
Article CAS PubMed PubMed Central Google Scholar
Huber, R., Ghilardi, M. F., Massimini, M. & Tononi, G. Local sleep and learning. Nature 430 , 78–81. https://doi.org/10.1038/nature02663 (2004).
Article ADS CAS PubMed Google Scholar
Tononi, G. & Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81 , 12–34. https://doi.org/10.1016/j.neuron.2013.12.025 (2014).
Feld, G. B., Bernard, M., Rawson, A. B. & Spiers, H. J. Sleep targets highly connected global and local nodes to aid consolidation of learned graph networks. Sci. Rep. 12 , 15086. https://doi.org/10.1038/s41598-022-17747-2 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Vidal, V. et al. Odor cueing during sleep improves consolidation of a history lesson in a school setting. Sci. Rep. 12 , 10350. https://doi.org/10.1038/s41598-022-14588-x (2022).
Zhang, J. et al. Evidence of an active role of dreaming in emotional memory processing shows that we dream to forget. Sci. Rep. 14 , 8722. https://doi.org/10.1038/s41598-024-58170-z (2024).
Gonzales, J. U., Dellinger, J. R. & Clark, C. Chronotype predicts working memory-dependent regional cerebral oxygenation under conditions of normal sleep and following a single night of sleep extension. Sci. Rep. 13 , 17897. https://doi.org/10.1038/s41598-023-45238-5 (2023).
Denisova, K. & Lin, Z. The importance of low IQ to early diagnosis of autism. Autism Res. 16 , 122–142 (2023).
Article PubMed Google Scholar
Denisova, K. Neurobiology of cognitive abilities in early childhood autism. JCPP Adv. https://doi.org/10.1002/jcv2.12214 (2024).
Article PubMed PubMed Central Google Scholar
Denisova, K. & Wolpert, D. Sensorimotor variability distinguishes early features of cognition in toddlers with autism. iScience 27 , 110685 (2024).
Song, C., Boly, M., Tagliazucchi, E., Laufs, H. & Tononi, G. fMRI spectral signatures of sleep. Proc. Natl. Acad. Sci. 119 , e2016732119. https://doi.org/10.1073/pnas.2016732119 (2020).
Article CAS Google Scholar
Song, C. & Tagliazucchi, E. Linking the nature and functions of sleep: Insights from multimodal imaging of the sleeping brain. Current Opin. Physiol. 15 , 29–36. https://doi.org/10.1016/j.cophys.2019.11.012 (2020).
Article Google Scholar
Quercia, A., Zappasodi, F., Committeri, G. & Ferrara, M. Local use-dependent sleep in wakefulness links performance errors to learning. Front. Hum. Neurosci. 12 , 122. https://doi.org/10.3389/fnhum.2018.00122 (2018).
Baglioni, C. et al. Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychol. Bull. 142 , 969–990. https://doi.org/10.1037/bul0000053 (2016).
Cooper, R., Di Biase, M. A., Bei, B., Quach, J. & Cropley, V. Associations of changes in sleep and emotional and behavioral problems from late childhood to early adolescence. JAMA Psychiatry 80 , 585–596. https://doi.org/10.1001/jamapsychiatry.2023.0379 (2023).
Rolling, J. et al. Sleep and circadian rhythms in adolescents with attempted suicide. Sci. Rep. 14 , 8354. https://doi.org/10.1038/s41598-024-57921-2 (2024).
Bauducco, S. et al. Adolescents’ trajectories of depression and anxiety symptoms prior to and during the COVID-19 pandemic and their association with healthy sleep patterns. Sci. Rep. 14 , 10764. https://doi.org/10.1038/s41598-024-60974-y (2024).
Reynolds, A. C. et al. Shift work, clinically significant sleep disorders and mental health in a representative, cross-sectional sample of young working adults. Sci. Rep. 12 , 16255. https://doi.org/10.1038/s41598-022-20308-2 (2022).
Wang, S. H. et al. Sleep problems during early and late infancy: Diverse impacts on child development trajectories across multiple domains. Sleep Med. 115 , 177–186. https://doi.org/10.1016/j.sleep.2024.02.018 (2024).
Gandal, M. J., Leppa, V., Won, H., Parikshak, N. N. & Geschwind, D. H. The road to precision psychiatry: Translating genetics into disease mechanisms. Nat. Neurosci. 19 , 1397–1407. https://doi.org/10.1038/nn.4409 (2016).
Berger, S. E. et al. The impact of extreme summer temperatures in the United Kingdom on infant sleep: Implications for learning and development. Sci. Rep. 13 , 10061. https://doi.org/10.1038/s41598-023-37111-2 (2023).
Correia, A. T. L. et al. Associations between fears related to safety during sleep and self-reported sleep in men and women living in a low-socioeconomic status setting. Sci. Rep. 14 , 3609. https://doi.org/10.1038/s41598-024-54032-w (2024).
Denisova, K. & Zhao, G. Inflexible neurobiological signatures precede atypical development in infants at high risk for autism. Sci. Rep. 7 , 11285. https://doi.org/10.1038/s41598-017-09028-0 (2017).
Download references
Acknowledgements
Denisova lab gratefully acknowledges support from the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH121605 (PI: Kristina Denisova), the Simons Foundation Autism Research Initiative (SFARI) under Award Number 614242 (PI: Kristina Denisova), and faculty start-up funds. Motomura lab gratefully acknowledges support from KAKENHI (24K02980) and faculty funds. Song lab gratefully acknowledges support from Wellcome Trust (209192/Z/17/Z) and European Commission (663830-CU119) and faculty funds. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The sponsors had no role in the writing of this paper, and in the decision to submit the paper for publication. The authors declare no conflicts of interest.
Author information
Authors and affiliations.
Division of Math and Natural Sciences, Department of Psychology, Autism Origins Lab, City University of New York, Queens College and Graduate Center, New York, NY, 10032, USA
Kristina Denisova
Department of Human Life Design and Science, Faculty of Design, Kyushu University, Fukuoka, 815-8540, Japan
Yuki Motomura
Cardiff University Brain Research Imaging Centre, School of Psychology, Cardiff University, Cardiff, CF24 4HQ, UK
You can also search for this author in PubMed Google Scholar

Contributions
KD, YM, and CS wrote paper.
Corresponding author
Correspondence to Kristina Denisova .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Denisova, K., Motomura, Y. & Song, C. Sleep on it: exploring the psychology of sleep amidst contemporary challenges. Sci Rep 14 , 30501 (2024). https://doi.org/10.1038/s41598-024-82330-w
Download citation
Published : 16 December 2024
DOI : https://doi.org/10.1038/s41598-024-82330-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sleep disruption
- Mental health
- Vulnerable populations
- Socioeconomic challenges
- Climate change
- Public health
- Social science
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Sleep preferentially consolidates negative aspects of human memory: Well-powered evidence from two large online experiments
Kristin e g sanders, elizabeth a kensinger, jessica d payne.
- Author information
- Article notes
- Copyright and License information
To whom correspondence may be addressed. Email: [email protected] .
Edited by Katharine Simon, University of California Irvine, Irvine, CA; received February 15, 2022; accepted May 24, 2022 by Editorial Board Member Henry L. Roediger III
Author contributions: D.D., E.A.K., and J.D.P. designed research; D.D. performed research; D.D. and K.E.G.S. analyzed data; D.D., K.E.G.S., E.A.K., and J.D.P. wrote the paper.
1 D.D. and K.E.G.S. contributed equally to this work.
Series information
Sleep, Brain, and Cognition Special Feature
Accepted 2022 May 24; Issue date 2022 Nov 1.
This article is distributed under Creative Commons Attribution-NonCommercial-NoDerivatives License 4.0 (CC BY-NC-ND) .
Significance
Recent research has called into question whether sleep improves memory, especially for emotional information. However, many of these studies used a relatively small number of participants and focused only on college student samples, limiting both the power of these findings and their generalizability to the wider population. Here, using the well-established emotional memory trade-off task, we investigated sleep’s impact on memory for emotional components of scenes in a large online sample of adults ranging in age from 18 to 59 y. Despite the limitations inherent in using online samples, this well-powered study provides strong evidence that sleep selectively consolidates negative emotional aspects of memory and that this effect generalizes to participants across young adulthood and middle age.
Keywords: sleep, emotion, memory
Research suggests that sleep benefits memory. Moreover, it is often claimed that sleep selectively benefits memory for emotionally salient information over neutral information. However, not all scientists are convinced by this relationship [e.g., J. M. Siegel. Curr. Sleep Med. Rep. , 7, 15–18 (2021)]. One criticism of the overall sleep and memory literature—like other literature—is that many studies are underpowered and lacking in generalizability [M. J. Cordi, B. Rasch. Curr. Opin. Neurobiol. , 67, 1–7 (2021)], thus leaving the evidence mixed and confusing to interpret. Because large replication studies are sorely needed, we recruited over 250 participants spanning various age ranges and backgrounds in an effort to confirm sleep’s preferential emotional memory consolidation benefit using a well-established task. We found that sleep selectively benefits memory for negative emotional objects at the expense of their paired neutral backgrounds, confirming our prior work and clearly demonstrating a role for sleep in emotional memory formation. In a second experiment also using a large sample, we examined whether this effect generalized to positive emotional memory. We found that while participants demonstrated better memory for positive objects compared to their neutral backgrounds, sleep did not modulate this effect. This research provides strong support for a sleep-specific benefit on memory consolidation for specifically negative information and more broadly affirms the benefit of sleep for cognition.
In thisspecial issue, which is devoted to sleep’s role in brain and cognitive function, it seems prudent to address head-on an issue that plagues not only sleep research ( 1 , 2 ) but also research more generally. Problems with statistical power and generalizability of findings cast doubt on even some of the most foundational research findings, leading to a replication crisis in cognitive neuroscience, psychology, medicine, and other fields ( 3 ). Sleep research is not immune to these problems ( 4 , 5 ). Therefore, it is now necessary to conduct large studies that move beyond homogeneous college participant samples to confirm key results, such as the essential role that sleep is believed to play in memory consolidation. Such studies are needed if we are to believe extant results and extend them to novel areas in our quest to understand the functions of sleep.
Of the many purported functions of sleep, its contribution to memory formation is one of the most important ( 6 ). Despite decades of research showing that sleep strengthens memory above and beyond wakefulness ( 7 , 8 ), sleep’s role in memory remains contested ( 1 ). Moreover, studies from different laboratories have shown that sleep prioritizes the consolidation of emotional over neutral memories, and to a larger degree than occurs across wake ( 9 – 13 ). However, recent reviews and meta-analyses have been unable to detect such effects when combining multiple studies, experimental designs, and task types ( 14 – 16 ). This raises the question of whether some of the prior results were spurious, arising from methodological flaws or publication bias rather than a true effect. An overreliance on small sample sizes in many experiments is likely a major contributor to conflicting results ( 2 ), with studies being underpowered to detect true effects. While it is challenging to recruit large samples for typical laboratory experiments, utilizing online tools to examine the behavioral effect of sleep on memory represents a promising avenue to increase sample size and sample from a broader portion of society ( 17 – 19 ). We turned to this resource to examine, in two well-powered experiments, whether sleep enhances emotional memory in human adults in the largest studies of sleep and emotional memory to date.
Emotion is a key indicator of important elements in our environment. As such, emotionally salient information is better remembered than neutral information. Within a single episode, more salient components of an experience are better remembered than the neutral context in which they occurred, known as an emotional memory trade-off effect ( 20 ). In a task often used to assess this effect, participants are presented with a series of scenes consisting of an object that is either emotional (e.g., a vicious-looking snake) or neutral (e.g., a harmless-looking chipmunk) on what is an always-neutral background (e.g., a forest scene). When tested, memories of the object and background components are examined separately. Participants typically remember emotionally negative objects better than neutral objects, but at the expense of the backgrounds on which they were presented. Thus, while one would remember the chipmunk and the forest equally well, the snake would be much better remembered than both the forest and the chipmunk.
In our research program, we have frequently shown how this disparity, or “trade-off,” between scene aspects increases over time, especially (and perhaps only) if sleep occupies the delay interval ( 21 – 26 ). As such, our prior research suggests that sleep is highly important, if not necessary, for the persistence of emotional selectivity during memory consolidation. Although in our laboratory we have studied the effects of sleep and the emotional memory trade-off in more than 11 studies utilizing various designs ( 21 – 33 ), sample sizes have been limited due to the laboratory-based nature of our experiments. Here, we more than doubled the sample size of not only our previous work in this area but also that of the broader sleep and memory field to robustly estimate and extend the generalizability of sleep’s impact on emotional memory.
Emotional memories can be positive as well as negative. However, much of the prior work in the sleep and emotional memory literature has examined only negatively valenced items, perhaps due to the more universally arousing and salient nature of negative items relative to neutral items( 34 – 36 ). Indeed, prioritization of emotional memories for subsequent sleep-based consolidation is theorized to be governed by salience and arousal cues present during encoding that tag the information for later processing during sleep ( 37 , 38 ). Prioritizing negative information may even be adaptive, allowing people to apply that learning to prevent and react to future negative scenarios ( 39 , 40 ). Nevertheless, positive memories can also be highly salient and arousing and can persist in the long-term, similarly to negatively valenced memories, and an emotional memory trade-off does occur for positive objects relative to neutral backgrounds ( 36 , 41 ). Therefore, in a second experiment, again using a large online sample, we examined whether sleep enhances memory for positive compared to neutral components of complex scenes.
We also addressed the issue of generalizability in the sleep and memory field. Many studies utilize college-student samples, a small subset of society that is typically more homogeneous than society more broadly ( 42 , 43 ). Because studies reporting a link between sleep and emotional memory typically employ such samples, it is unclear whether we can extrapolate such findings to the wider population. One important dimension of generalizability is how consistent the effects of sleep on emotional memory are across the life span. Aging is characterized by marked changes in sleep quality and physiology, which are implicated in changes in and deterioration of sleep-based memory consolidation ( 44 – 52 ). By older age, there also can be a diminution of negative memory biases and a shift toward positive memory ( 53 , 54 ), a shift that is also seen in sleep-based memory processing ( 55 ). Therefore, it is crucial that a broad age range is considered when asking the question of whether sleep preferentially consolidates emotional information. In the present study, we included young through middle-aged adults (age range 18 to 59 y) since middle-aged individuals constitute a particularly underresearched group ( 56 ), even though changes in both sleep and emotional memory processing are already evident by middle age ( 57 , 58 ). The large sample also allowed us to explore other potential demographic moderators of the sleep–emotional memory relationship, such as biological sex, income, and minority status.
We performed two experiments examining the effect of sleep on the emotional memory trade-off effect. In each experiment, we varied the valence of emotional items that participants were exposed to. In Experiment 1, participants viewed either negative or neutral scenes to investigate whether the original sleep-based enhancement of the emotional memory trade-off effect ( 21 ) could be replicated in a large sample. In Experiment 2, we compared positive and neutral scenes to determine if sleep also enhances memory for positive information. With over 250 participants in each study, recruited from a wide sample of participants across the United States, these are, to our knowledge, the largest studies in human adults to compare the effects of sleep and wake on memory generally, let alone emotional memory specifically.
Experiment 1.
A total of 280 participants completed both sessions of Experiment 1. Full participant demographics can be found in SI Appendix , Table S1 . We recruited participants in four age groups (18 to 24, 25 to 35, 36 to 47, and 48 to 59 y old) to adequately sample participants across young and middle adulthood. After obtaining informed consent, participants within each age range were randomly assigned to a sleep ( n = 141) or wake ( n = 139) delay condition before participating in two experimental sessions ( Fig. 1 A ). In the first session, participants viewed a series of 64 scenes in either the morning or evening. Half of these scenes (32) contained a negative object superimposed on a neutral background, while the other 32 contained a neutral object superimposed on a neutral background. Participants were instructed to rate each scene for its perceived valence and arousal and were not told about the later memory test ( Fig. 1 B and SI Appendix , Table S2 ). Participants rated scenes as significantly more negative [ F (1, 263) = 1,408, P < 0.001, ηp 2 = 0.84] and significantly more arousing [ F (1, 267) = 960, P < 0.001, ηp 2 = 0.78]. All other main effects and interactions were nonsignificant (all P > 0.10). Age did not correlate with participants’ ratings (all P > 0.09), demonstrating that valence and arousal ratings did not vary according to time of day or participant age ( SI Appendix , Table S3 presents full ANOVA tables).
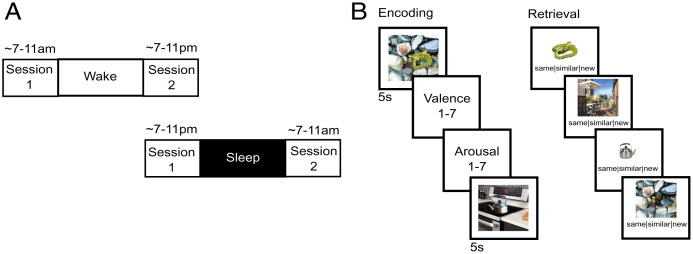
Experimental design. ( A ) Study timeline. Both experiments followed the same general protocol. After completing a screening survey, participants were randomly assigned to a sleep or wake delay condition. Participants in the Sleep condition completed Session 1 in the evening and Session 2 the following morning. Participants in the Wake condition completed Session 1 in the morning and Session 2 later that evening. In Session 1, participants performed the encoding portion of the emotional memory trade-off task. In Session 2, participants came back to perform the surprise recognition portion. ( B ) Emotional memory trade-off task. During encoding, participants viewed a series of scenes containing an object placed on a background and were asked to rate each scene for its valence and arousal. Each scene appeared on the screen for 5 s, after which participants made their valence and arousal judgments. During recognition, participants viewed scene components individually and one at a time. Some of these components were identical to components of scenes viewed during Session 1, others were similar in visual detail but not an identical match, and others were new images. For each trial, participants had to judge whether the item was the same or similar to a component viewed in Session 1 or if it was a new item.
Approximately 12 h later, following a delay containing either subjectively reported nocturnal sleep or daytime wakefulness, participants returned for a second experimental session. Participants in the sleep group self-reported sleeping an average of 7.43 h ( SD = 1.63). During this session, participants performed a surprise recognition test in which objects and backgrounds were presented separately one at a time ( Fig. 1 B ). For each item, participants indicated whether the item 1) was an exact match to a previously viewed component (“same”), 2) shared the same verbal label as a previously viewed component but was not an exact match (“similar”), or 3) was not seen before (“new”). Overall recognition was calculated as a corrected recognition score (hits − false alarms), where a hit was scored as responding “same” or “similar” to a same item, and a false alarm was scored as saying “same” to a new item ( 32 ). We also calculated a more stringent, corrected specific recognition score where a hit constituted responding “same” to a same item. A trade-off magnitude score was created for each recognition score by subtracting the background score from the object score, separately for negative and neutral items (see Materials and Methods for full details). Component hit and false alarm rates are displayed in SI Appendix , Table S4 . Our primary analysis consisted of a 2 (valence: negative, neutral) × 2 (component: object, background) × 2 (delay: sleep, wake) mixed ANOVA.
Critically, we found that sleep selectively enhanced the emotional memory trade-off effect in overall recognition, characterized by a significant three-way interaction between emotion, component, and delay condition [ F (1, 278) = 4.12, p adj = 0.043, ηp 2 = 0.02; Fig. 2 and SI Appendix , Table S5 ]. This suggests that participants’ memory for negative relative to neutral information varied depending on whether the delay period was filled with sleep or wake. Indeed, follow-up tests found that participants remembered negative objects better after a night of sleep than after a day of wakefulness [ t (275) = 2.14, P = 0.034, d = 0.26; Fig. 2] , but there were no sleep-wake differences for neutral objects or either paired backgrounds (all P > 0.310). This preferential sleep-based enhancement of negative object memory translated into a significantly larger trade-off magnitude score (objects − backgrounds) in the sleep group for negative [ t (277) = 2.73, P = 0.007, d = 0.33] but not neutral items [ t (277) = 0.47, P = 0.469, d = 0.06; Fig. 3 A ] .
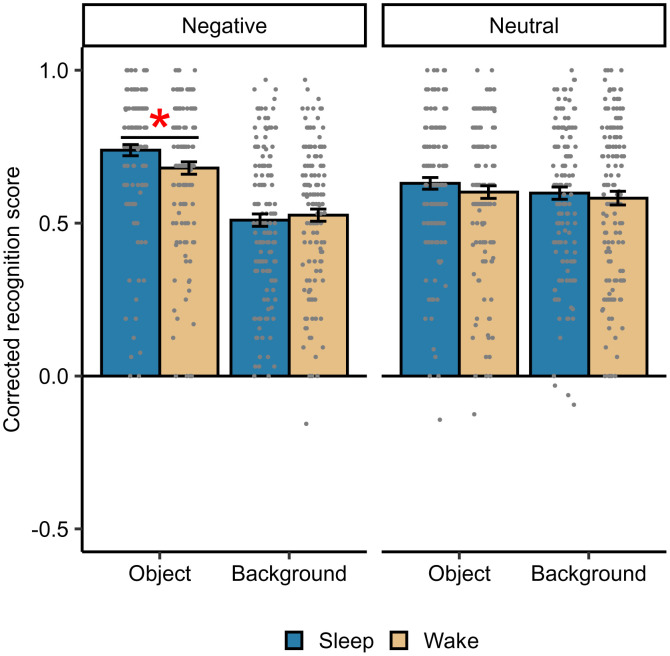
Sleep enhanced the negative components of memory in a sample of 280 adults ranging in age from 18 to 59 y. Overall corrected recognition memory for objects and backgrounds followed either a sleep or wake delay. Negative objects were remembered better after sleep than after wakefulness. This was the only significant difference. All other sleep-wake differences were not significant. *, P < 0.05. Error bars are between-subjects SEMs.
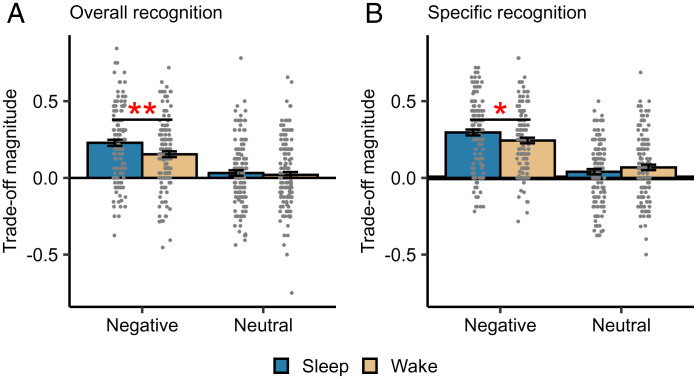
Magnitude of the trade-off effect (object memory − background memory) following either a sleep or wake delay. We found a significant increase in the negative trade-off magnitude after sleep for both overall and specific recognition memory. Sleep and wake did not differ in the magnitude of the trade-off for neutral items. ( A ) Trade-off magnitude for overall recognition memory. ( B ) Trade-off magnitude for specific recognition memory. **, P < 0.01; *, P < 0.05. Error bars are between-subjects SEMs.
Specific recognition memory followed the same pattern as overall recognition. We again found a significant three-way interaction between emotion, component, and delay condition [ F (1, 278) = 6.79, p adj = 0.020, ηp 2 = 0.02] such that the trade-off magnitude was larger for the sleep group than for the wake group for negative [ t (278) = 2.00, P = 0.047, d = 0.24] but not neutral items [ t (278) = 1.15, P = 0.250, d = 0.14; Fig. 3 B ] .
This result confirms that sleep enhanced the emotional memory trade-off effect in a large online sample. Given that generalizability was a key aim here, we next looked in more detail at whether the interaction between sleep and emotional memory trade-off was further moderated by age, sex, income, and minority status. With regard to age, we did not observe a significant four-way interaction between emotion, component, delay, and age [ F (3, 272) = 0.14, p adj = 0.935, ηp 2 = 0.002]. Correlations between age and full trade-off magnitude (negative trade-off minus neutral trade-off) were not significant across a wake ( r = −0.13, p adj = 0.226) or sleep delay ( r = −0.05, p adj = 0.539). We also note that no four-way interaction between emotion, component, delay, and age was observed when age was treated as a binary variable in two broader age bins [younger (18 to 35 y) versus older (36 to 59 y; P = 0.95], and the three-way interaction between emotion, component, and delay remained significant when age was included as a continuous covariate ( P = 0.044). As such, we are quite confident that sleep’s enhancing effect on the emotional memory trade-off effect is preserved across middle age.
Similarly, the effect of sleep on the emotional trade-off did not vary reliably by biological sex [male or female; F (1, 275) = 4.33, p adj = 0.152, ηp 2 = 0.02], nor did age and sex interactively predict trade-off magnitude in either the sleep [ F (3, 132) = 0.294, P = 0.829, ηp 2 = 0.007] or wake [ F (3, 131) = 0.788, P = 0.503, ηp 2 = 0.01] conditions. The impact of sleep on the trade-off did not vary reliably by minority status [minority or nonminority; F (1, 276) = 2.45, p adj = 0.238, ηp 2 = 0.01] or income [≥ median income versus < median income; F (1, 257) = 1.19, p adj = 0.368, ηp 2 = 0.01], and income did not correlate with full trade-off magnitude across either a wake ( r = −0.18, p adj = 0.079) or sleep delay ( r = −0.01, p adj = 0.878).
In summary, Experiment 1 confirmed that sleep improved negative emotional memory in a large online sample. Participants remembered negative objects better after a night of sleep than an equivalent period of time spent awake. Thus, sleep amplified the emotional memory trade-off effect. This effect was found to be consistent across a wide age range and did not vary by sex, income, or minority status. As such, this experiment shows that sleep selectively enhances the negative components of memory, an effect that can be seen in a large sample spanning young through middle age.
Experiment 2.
In Experiment 2, we investigated whether sleep would similarly enhance positive memory. In a second large online experiment, we recruited a separate sample of 264 participants (129 sleep, 135 wake), who performed the same experimental protocol as in Experiment 1 except that the negative scenes were replaced with positive scenes. Between sessions, participants in the sleep group self-reported sleeping 7.44 h ( SD = 1.42). When examining participants’ ratings of the scenes during the encoding session, we found a main effect of emotion on valence ratings [ F (1, 243) = 115, P < 0.001, ηp 2 = 0.32] and a significant main effect of emotion on arousal ratings [ F (1, 248) = 159, P < 0.001, ηp 2 = 0.39], with positive scenes being rated as significantly more positive and more arousing than neutral scenes ( SI Appendix , Table S2 ). There were no differences in ratings between the sleep and the wake conditions, suggesting that participants’ ratings did not vary by the time of day ( SI Appendix , Table S3 ). We found that participants’ ratings of positive scenes increased with age ( r = 0.18, P = 0.006) such that older adults tended to rate positive scenes more positively, but there were no other correlations between age and valence or arousal measures.
To test whether sleep enhanced positive emotional memory, we again conducted a three-way ANOVA including emotion, component, and delay as factors and controlling for age. Unlike in Experiment 1, we did not find a significant three-way interaction for either overall recognition [ F (1, 262) < 0.01, p adj = 0.987, ηp 2 < 0.01; Fig. 4 A ] or specific recognition [ F (1, 262) =0.3387, p adj = 0.987, ηp 2 =0.01]. Similarly, we did not find significant sleep-wake differences in either the positive or neutral overall trade-off magnitudes [ t (256) = 0.13, P = 0.902, d = 0.02; t (257) = 0.11, P = 0.910, d = 0.01, respectively; Fig. 4 B ] . The trade-off magnitudes also did not differ for specific recognition [ t (253) = 0.15, P = 0.885, d = 0.02 and t (256) = 0.92, P = 0.361, d = 0.11 for positive and neutral trade-off magnitudes, respectively].
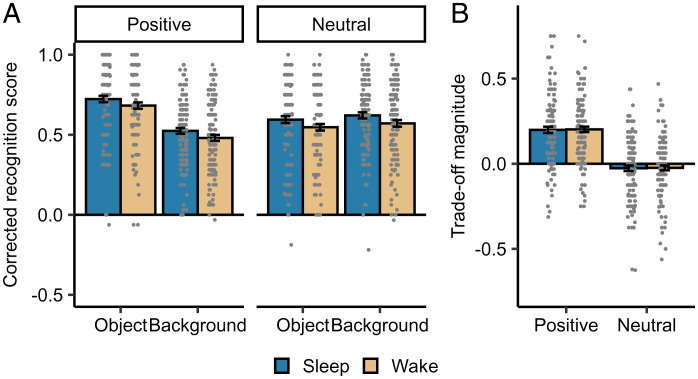
Sleep does not enhance the positive components of memory. ( A ) Overall recognition memory for objects and backgrounds following either a sleep or wake delay. ( B ) Magnitude of the trade-off effect for positive and neutral items in Experiment 2. Errors bars are between-subjects SEMs.
We further confirmed that the lack of a difference between the sleep and wake conditions was not due to a lack of trade-off effect overall. The two-way interaction between emotion and component was highly significant [overall: F (1, 262) = 202.11, P < 0.001, ηp 2 = 0.44; specific: F (1, 262) = 152.32, P < 0.001, ηp 2 = 0.37]. In fact, when directly comparing the magnitude of the positive memory trade-off effect, regardless of delay condition, in Experiment 2 with the magnitude of the negative memory trade-off effect in Experiment 1, we did not find a statistical difference between the positive and negative trade-off effects [ t (537) = 0.48, P = 0.630, d = 0.04].
We next directly compared sleep-associated processing of negative and positive stimuli by running a 2 (emotion: negative, positive) × 2 (delay condition: sleep, wake) ANOVA on trade-off magnitudes across the two experiments. A significant emotion * delay condition interaction [ F (1, 540) = 4.47, P = 0.035, ηp 2 = 0.008] confirmed that the effect of sleep on the emotional memory trade-off existed for negative but not positive stimuli. When compared within delay condition, the negative and positive trade-off effect was equivalent for the sleep group [ t (268) = 1.10, P = 0.272, d = 0.13], whereas the negative trade-off was marginally smaller than the positive trade-off in the wake condition [ t (267) = 1.94, P = 0.053, d = 0.23]. We then tested whether an effect of sleep on the positive memory trade-off would reveal itself if we restricted our analysis to only the most highly arousing positive items. To do this, we divided positive scenes into either high or low arousal based on a median split of a participant’s arousal ratings. We did not see a significant difference in trade-off magnitude between high and low arousal scenes [ F (1, 262) = 0.40, P = 0.526, ηp 2 = 0.002], nor was there a significant interaction between scene arousal and delay condition [ F (1, 262) = 1.74, P = 0.19, ηp 2 = 0.007].
The positive memory trade-off and its relation to delay condition did not interact with age, sex, income, or minority status (all p adj > 0.34), suggesting results were generalizable across the whole sample demographic. Together, these results suggest that sleep does not selectively enhance positive emotional memories.
The results of the two experiments together demonstrate that sleep selectively enhances negative, but not positive, emotional memories. Furthermore, the lack of a sleep effect for positive memories was not due to a lack of a positive memory trade-off overall. In fact, the size of the positive trade-off magnitude was similar to that of the negative trade-off magnitude in Experiment 1. However, there was no evidence of a sleep-specific enhancement of the positive components of memory in Experiment 2.
Alertness Measures and Subjective Sleep Quality.
There were no main effects or interactions between sessions and delay conditions for the psychomotor vigilance test (PVT) reaction time in either experiment ( SI Appendix , Table S6 ). As such, our differences between the sleep and wake groups could not be attributed to differences in alertness during either the encoding or recognition portions of the experiment. Subjective sleep quality over the month prior to participation, the three nights prior to the experiment, and during the delay between encoding and retrieval (sleep group only) are displayed in SI Appendix , Tables S7–S9 . We did not observe any consistent correlations between subjective sleep parameters and the magnitude of the trade-off effect. See Materials and Methods for full details of measures obtained.
Our results clearly demonstrate that sleep selectively enhances the negative components of memory relative to an equivalent period of time spent awake. These results advance the field of sleep and memory in three important ways. First, this study is the largest-ever replication of sleep’s effect on emotional memory in human adults; moreover, it presents direct counterevidence to recent meta-analyses that suggest sleep does not play a role in emotional memory ( 14 – 16 ). Second, our results show that the sleep-specific enhancement of the emotional memory trade-off effect generalizes beyond college student samples and persists into middle age. Finally, we show that sleep and wake do not differ in memory for positive emotional memories, suggesting that sleep’s preferential effect is specific to negative emotional memories.
Issues of replicability are a major concern in all areas of psychological research, and sleep and memory is no exception. Recent meta-analyses and reviews have cast doubt on the relationship between sleep and emotional memory, showing that the majority of studies do not suggest a prioritization of emotional information during sleep over and above what occurs across wake ( 14 – 16 ). A major driver of replicability issues is low statistical power, with the risk of spurious findings being higher in small sample studies that also likely overinflate true effect sizes ( 59 , 60 ). With these issues in mind, the present research represents an important step forward in our understanding of the link between sleep and emotional memory. Confirming our previous laboratory studies using the same task ( 21 – 26 ), we confirmed in a far larger and broader sample that sleep selectively strengthens the negative components of memory, leading to a larger emotional memory trade-off effect than occurs over an equivalent period of wake.
Compared to other investigations of sleep and emotional memory that have utilized different tasks (e.g., 10 , 12 , 13 , 55 , 61 ), the emotional memory trade-off task may be particularly suited to measuring sleep’s role in emotional memory processing. Our outcome measures directly compared memory within a single experience (the negative objects compared to their neutral backgrounds) as opposed to discrete stimuli. This represents a more naturalistic task with clear real-world analogues. For example, in eyewitness identification, the presence of a threatening weapon distracts eyewitnesses from characteristics of the perpetrator (i.e., the weapon focus effect). In other words, memory for the negatively salient object (the weapon) is preserved at the expense of other visual details [specific memory for details of the perpetrator’s face ( 62 )]. The fact that the trade-off task parallels nuanced memory phenomena in the real world may allow the relative sleep enhancement of negative memory to be clearly demonstrated. Perhaps this is why the majority of studies using the trade-off task, including this one, confirm sleep’s selective benefit for negative information ( 15 , 21 – 26 ).
Another strength of the current study is its broad population sample. Compared to laboratory studies that generally draw from college-educated young adults in a single location, our online study recruited participants from a much wider range of ages (18 to 59 y), locations across the United States, and education levels. We deliberately chose few inclusion and exclusion criteria in order to further broaden the sample. Replicating the effect of sleep on the emotional memory trade-off effect in this study suggests that the effect generalizes beyond the typical laboratory sample. The effect size for the sleep-wake difference in emotional memory trade-off magnitudes in this sample was smaller ( d = 0.32) than in previous laboratory studies. This may represent a closer approximation of the actual size of the effect, or it may reflect the cost of decreased experimental control that occurs in online studies. However, the fact that our replication was successful under much less stringent experimental conditions in itself speaks to the strength of the findings. A combination of online research, which can easily facilitate large sample sizes to establish behavioral effects, followed by detailed laboratory work to determine physiological mechanisms would allow the field to move forward in both directions. We do note that this study was primarily interested in generalizability across different ages; thus, some critical areas of inclusion were not addressed. For example, the study population was relatively homogeneous in terms of race and ethnicity, and participants were recruited from just a single country. It will be important in future work to address these disparities.
Although we found a robust effect of sleep on negative emotional memory, Experiment 2 provided no evidence that sleep similarly benefits positive memory. This lack of effect is unlikely to be driven by the size of the emotional memory trade-off, since we did not find that the overall negative and positive trade-off magnitudes differed between the two experiments. One possibility is that different aspects of the emotional experience at encoding underlie the sleep effect. We have argued that at initial exposure, important information is tagged for later processing during sleep ( 37 ). Therefore, the initial encoding experience is crucial to eliciting the later sleep effect. Arousal-based neuromodulators such as norepinephrine and cortisol may act as such tags, with arousal during encoding being an important predictor for subsequent consolidation during sleep ( 63 , 64 ). In the present study, positive scenes were consistently rated as less arousing than negative scenes. As such, the lack of a sleep effect for positive items adds evidence to the idea that emotional memory processing during sleep may be guided by arousal during encoding. However, when we focused our analyses on only the most highly arousing positive images, we still did not detect a significant effect of sleep. Different neural systems underlie the processing of negative and positive emotional experiences ( 65 ). Sleep may preferentially act upon the negative system for evolutionary benefit. Selectively honing in on potentially threatening aspects of an experience may allow such experiences to be strongly preserved in memory for future scenarios ( 66 ). It is unclear from this study whether encoding both positive and negative items within the same experience would lead to a generalized sleep effect for all emotional items or if one particular valence might “win out,” perhaps guided by arousal and reactivity to the stimuli themselves, as well as personal salience and relevance cues. This will be an important next step for research on emotional memory and sleep.
This study employed the “classic” sleep and memory design of contrasting memory after a day of wakefulness versus a night of sleep. We were motivated to use this design given its widespread use in the literature (e.g. 10 , 11 , 13 , 55 , 61 ) and the fact that the study we aimed to replicate also used this design ( 21 ). Although there are several limitations to this approach, we believe that our prior body of work addressed these limitations. First, it is possible that differences in memory performance are due to circadian influence. However, we previously showed that there are no time-of-day differences in memory for the trade-off task after short 30-min delays in the morning or evening ( 21 ). Similarly, we found a sleep-enhancing effect on the emotional memory trade-off in afternoon nap studies, where time of training and testing was equivalent across groups ( 24 , 25 ). Additionally, in the present work, participants were randomly assigned to the sleep or wake condition, so they could not self-select based on circadian preference. PVT scores also show the groups were similarly alert during the evening and morning sessions.
Second, the wake group would have encountered more potentially interfering information during the delay than the sleep group, meaning that the sleep benefit may simply be due to passive protection from interference. While this behavioral-only study cannot identify the exact mechanism(s) underlying the sleep effect, we point to other work from our group using a 24-h delay design, where participants spent an equal amount of time awake and asleep. Negative emotional memory benefits are larger when sleep comes first, compared to when wake occurs first ( 23 ). This argues in favor of sleep actively stabilizing memories, making them more resilient from subsequent interference. If sleep merely provided passive protection against interference, then we would expect memory to be equivalent regardless of whether sleep comes soon after learning or after a day of wakefulness. We have also demonstrated that negative emotional memories are strongly positively correlated with time spent in rapid eye movement sleep, but not other sleep stages (indicating a potential overnight sleep mechanism) and, importantly, not with total sleep time ( 23 ). Together, these results suggest that aspects of sleep actively stabilize emotional aspects of memories. This is not to say that protection from interference plays no role in the sleep effect at all—indeed, we expect that it does. It does argue, however, that in addition to passive protection, sleep also has unique properties that actively promote memory consolidation ( 37 , 67 , 68 ).
Finally, the 12-h interval between encoding and retrieval may not be long enough for some effects to develop. Our previous studies using this task found that the trade-off effect was apparent almost immediately after testing, but got larger over time ( 23 ). Sleep benefits can be seen with as little as a 6-h delay ( 24 ) and as long as a 24-h delay ( 23 ). Whether the sleep-specific enhancement of the trade-off effect persists over even longer delays remains to be seen. Other work, however, has found that the benefits of sleep on emotional memory can be seen years later ( 69 ). While it is possible that an effect of sleep on positive memory may have been observed if a longer delay was used, our data cannot speak to such a possibility. We found that the strength of the positive trade-off was marginally stronger across wake compared to the negative trade-off. Although speculative, perhaps the positive components of memory were more strongly encoded than the negative components, which would render these stronger memories less susceptible to decay over time and thus make it harder to see a benefit of sleep.
The question of whether sleep selectively enhances emotional memory over and above wakefulness remains a hotly contested question. Small sample sizes derived mostly from college students remain as limitations in the sleep and memory literature. Here, we tackled both of these limitations head-on. We provide strong evidence that sleep selectively enhances negative emotional experiences. Furthermore, our results broadly support a role for sleep in memory and cognition, lending credence to the necessity of a good night’s sleep.
Materials and Methods
Participants..
For both experiments, participants were recruited online via Prolific ( https://www.prolific.co ). The study was described as an investigation into “emotional reactivity at different times of day” to obscure the later memory test. In order to ensure we obtained similar numbers of participants across a broad age range, participants were recruited in four separate postings that were made visible to the following age brackets: 18 to 24, 25 to 35, 36 to 47, and 48 to 59 y old. After undergoing informed consent, prospective participants filled out a screening survey to determine their eligibility. Inclusion criteria were age between 18 and 59 y, currently residing in the United States, fluent in English, having a Prolific approval rating of at least 85, and free of any diagnosed sleep, psychiatric, or neurological disorders. After filling out the screening form, eligible participants were immediately invited to participate in the main part of the study. Participants assigned to the sleep condition were invited to perform part 1 of the study that same evening (and part 2 the following morning), while wake participants were invited to perform part 1 the next morning and part two later that evening. This exact recruitment and condition assignment procedure was followed for both experiments.
For Experiment 1, a total of 554 eligible participants were recruited. Eligible participants were then randomly assigned to a sleep or wake delay condition. Of those eligible, 280 participants completed both experimental sessions and were included in the final analysis (sleep: 141; wake: 139). A power analysis determined that a sample size of 280 participants yielded 80% power to detect a significant difference between sleep and wake at P < 0.05 with an effect size of d = 0.33.
For Experiment 2, 533 eligible participants were recruited and randomly assigned to a sleep or wake delay condition. Of these, 264 completed both experimental sessions and were included in the final analysis (sleep: 129; wake: 135).
In all experiments, participants were financially compensated for their time spent completing the screening survey regardless of final eligibility status. Eligible participants then received further payments for completing each experimental session. The study was approved by the University of Notre Dame Internal Review Board. Full participant demographics from all experiments can be found in SI Appendix , Table S1 .
Emotional memory trade-off task.
The studied materials consisted of a series of scenes depicting negative ( n = 32), positive ( n = 32), or neutral objects ( n = 32) placed on plausible, always neutral backgrounds 32. Two versions of each scene were created using two similar objects and backgrounds. Online pilot testing in n = 30 participants confirmed significant differences between negative, positive, and neutral scenes in terms of subjective valence and arousal ratings. A further 48 objects (16 negative, 16 positive, and 16 neutral) and 32 neutral backgrounds served as new items during the recognition test.
The PVT is a standardized measure of alertness ( 70 ). In the version utilized in the present study, participants were instructed to press the space bar as quickly as possible every time a red dot appeared on their computer screen but were told not to press the button too soon (i.e., before the dot appeared on the screen) ( 71 ). The interstimulus interval varied randomly from 1 to 4 s. To reduce participant burden, we used the brief form of the PVT, which lasted for a total of 3 min ( 72 ).
Fig. 1 A shows a schematic of the study timeline. The procedure was identical for both experiments, with the exception of which stimuli were used. All participants completed two experimental sessions. Participants assigned to the sleep delay condition performed Session 1 in the evening (between 7 and 11 PM at the participant’s local time) and completed Session 2 12 h later the following morning (7 to 11 AM at the participant’s local time). Participants in the wake condition performed Session 1 in the morning (7 to 11 AM) and Session 2 12 h later in the evening (7 to 11 PM).
During Session 1, participants first completed a battery of questionnaires to assess subjective sleep and well-being using the Qualtrics survey platform. Participants’ sleep quality over the month prior to the experiment was assessed using the Pittsburgh Sleep Quality Index ( 73 ), and they retrospectively completed a 3-d sleep diary to characterize their sleep in the days prior to the experiment. They then completed the PVT to assess current alertness levels before performing the encoding portion of the emotional memory trade-off task. During encoding, participants viewed a series of scenes for 5,000 ms each. Participants viewed 64 scenes in total (Experiment 1: 32 negative scenes, 32 neutral scenes; Experiment 2: 32 positive scenes, 32 neutral scenes). For each scene, participants were instructed to rate the scene for its valence and arousal. Valence was rated on a 1 to 7 scale where 1 = very negative, 4 = neutral, and 7 = very positive. Arousal was rated on a second 1 to 7 scale where 1 = highly calming/subduing, 4 = neither calming/subduing nor agitating/exciting, and 7 = high agitating/exciting. After completing Session 1, participants in the wake group were told to refrain from napping during the delay interval. No other specific instructions were given.
When participants returned for Session 2, they completed additional questionnaires before completing a second PVT assessment. Sleep participants completed another sleep diary to measure subjective sleep between experimental sessions. Sleep participants also answered questions relating to their sleep inertia that morning, using a modified version of the sleep inertia questionnaire ( 74 ). They then performed an unexpected, self-paced recognition task in which objects and backgrounds were presented separately and one at a time. Some of these objects and backgrounds were identical to the scene components that had been viewed during Session 1, others were the alternate version of the object or background and thus shared the same verbal label but differed in specific visual details, and others were objects or backgrounds that had not been seen during Session 1. Participants saw either the same or a similar version of a particular item during the recognition test, never both. For each item, participants indicated whether it was an exact match to a previously viewed component (same), similar but not an exact match (similar), or not seen before (new). In Experiment 1, the recognition task included 32 same objects (16 negative, 16 neutral), 32 similar objects (16 negative, 16 neutral), 32 new objects (16 negative, 16 neutral), 32 same backgrounds (16 initially paired with a negative object, 16 initially paired with a neutral object), 32 similar backgrounds (16 negative, 16 neutral), and 32 new backgrounds. The trial count was identical in Experiment 2, except positive stimuli replaced the negative stimuli. The experimental tasks were hosted on the Cognition.run platform ( https://www.cognition.run ) and were programmed using jsPsych ( 75 ).
To investigate the effects of sleep and age on memory at the recognition test, we calculated an overall corrected recognition score. For this measure, a “hit” was defined as saying either “same” or “similar” to a same trial, and a false alarm was considered as saying “same” to a new trial ( 32 ). We calculated the proportion of hits and false alarms and then subtracted the false alarm rate from the hit rate to obtain an overall corrected recognition score. Consistent with prior studies, this score reflected at least partial memory for the studied scene. That is, for a same item to be identified as either same or similar, participants had to remember that at least a particular type of object or background had been originally studied, because otherwise they would have indicated the item to be new. We also calculated a specific recognition score where hits were defined as saying “same” to a same trial, reflecting precise memory for the studied item. We then subtracted the false alarm rate from the specific recognition hit rate to form a corrected specific recognition score. A corrected recognition score was calculated for each scene component (object, background) and valence (negative, positive, neutral, depending on experiment).
To quantify the magnitude of the trade-off effect (that is, memory for objects relative to the backgrounds they were presented on), we subtracted the corrected recognition score for backgrounds from the corrected recognition score for objects. (separately for negative, positive, and neutral scenes). Here, a positive value would indicate better memory for objects relative to their paired backgrounds, whereas a negative value would suggest better memory for backgrounds relative to their paired objects.
To answer our primary question regarding the effects of sleep on the emotional memory trade-off effect, we performed two 2 (valence: emotional, neutral) × 2 (component: object, background) × 2 (delay: sleep, wake) mixed ANOVAs with either overall or specific corrected recognition scores as the dependent variables. We considered a false discovery rate (FDR)–adjusted P < 0.05, with significance adjusted for two tests (overall and specific recognition), to be statistically significant. Follow-up tests were performed as appropriate.
To assess potential moderators of the sleep and emotional memory trade-off effect, we conducted further mixed ANOVAs to investigate whether demographic variables interacted with the sleep and trade-off effect. Specifically, models were run to examine age: 2 (valence) × 2 (component) × 2 (delay) × 4 (age: 18 to 24 y, 25 to 35 y, 36 to 47 y, 48 to 59 y); biological sex: 2 (valence) × 2 (component) × 2 (delay) × 4 (sex: female, male); income: 2 (valence) × 2 (component) × 2 (delay) × 4 (income: ≥ sample median, < sample median); and minority racial status: 2 (valence) × 2 (component) × 2 (delay) × 4 (minority, nonminority). FDR-adjusted P < 0.05, with significance adjusted for four tests, was considered to be statistically significant. We also ran Pearson correlations between the magnitude of the full trade-off effect (emotional trade-off minus neutral trade-off) with age and income level, with P values again being adjusted for multiple comparisons using FDR. Uncorrected correlations between full trade-off magnitude and sleep variables are shown in SI Appendix , Tables S7–S9 .
Supplementary Material
Acknowledgments.
This work was supported by the National Science Foundation under Grant BCS-2001025, awarded to J.D.P. (PI) and E.A.K. (co-PI).
The authors declare no competing interest.
This article is a PNAS Direct Submission. K.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2202657119/-/DCSupplemental .
Data Availability
Anonymized data (i.e., to reproduce reported results) have been deposited in Open Science Framework ( 10.17605/OSF.IO/24Q6M ) ( 76 ).
- 1. Siegel J. M., Memory consolidation is similar in waking and sleep. Curr. Sleep Med. Rep. 7, 15–18 (2021). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Cordi M. J., Rasch B., How robust are sleep-mediated memory benefits? Curr. Opin. Neurobiol. 67, 1–7 (2021). [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Baker M., 1,500 Scientists lift the lid on reproducibility. Nature 533, 452–454 (2016). [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Pöhlchen D., Pawlizki A., Gais S., Schönauer M., Evidence against a large effect of sleep in protecting verbal memories from interference. J. Sleep Res. 30, e13042 (2020). [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Bailes C., Caldwell M., Wamsley E. J., Tucker M. A., Does sleep protect memories against interference? A failure to replicate. PLoS One 15, e0220419 (2020). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Born J., Wilhelm I., System consolidation of memory during sleep. Psychol. Res. 76, 192–203 (2012). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Rasch B., Born J., About sleep’s role in memory. Physiol. Rev. 93, 681–766 (2013). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Diekelmann S., Born J., The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 (2010). [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Prehn-Kristensen A., et al. , Sleep promotes consolidation of emotional memory in healthy children but not in children with attention-deficit hyperactivity disorder. PLoS One 8, e65098 (2013). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Nishida M., Pearsall J., Buckner R. L., Walker M. P., REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb. Cortex 19, 1158–1166 (2009). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Hu P., Stylos-Allan M., Walker M. P., Sleep facilitates consolidation of emotional declarative memory. Psychol. Sci. 17, 891–898 (2006). [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Wagner U., Gais S., Born J., Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn. Mem. 8, 112–119 (2001). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Cox R., et al. , Sleep selectively stabilizes contextual aspects of negative memories. Sci. Rep. 8, 17861 (2018). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Lipinska G., Stuart B., Thomas K. G. F., Baldwin D. S., Bolinger E., Preferential consolidation of emotional memory during sleep: A meta-analysis. Front. Psychol. 10, 1014 (2019). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Davidson P., Jönsson P., Carlsson I., Pace-Schott E., Does sleep selectively strengthen certain memories over others based on emotion and perceived future relevance? Nat. Sci. Sleep 13, 1257–1306 (2021). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Schäfer S. K., et al. , Sleep’s impact on emotional recognition memory: A meta-analysis of whole-night, nap, and REM sleep effects. Sleep Med. Rev. 51, 101280 (2020). [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Kroneisen M., Kuepper-Tetzel C. E., Using day and night—Scheduling retrieval practice and sleep. Psychol. Learn. Teach. 20, 40–57 (2021). [ Google Scholar ]
- 18. Ashton J. E., Cairney S. A., Future-relevant memories are not selectively strengthened during sleep. PLoS One 16, e0258110 (2021). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Ashton J. E., Staresina B. P., Cairney S. A., Sleep bolsters schematically incongruent memories. PLOS ONE , 17 , e0269439 (2022). [ DOI ] [ PMC free article ] [ PubMed ]
- 20. Kensinger E. A., Garoff-Eaton R. J., Schacter D. L., Memory for specific visual details can be enhanced by negative arousing content. J. Mem. Lang. 54, 99–112 (2006). [ Google Scholar ]
- 21. Payne J. D., Stickgold R., Swanberg K., Kensinger E. A., Sleep preferentially enhances memory for emotional components of scenes. Psychol. Sci. 19, 781–788 (2008). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Payne J. D., Kensinger E. A., Sleep leads to changes in the emotional memory trace: Evidence from FMRI. J. Cogn. Neurosci. 23, 1285–1297 (2011). [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Payne J. D., Chambers A. M., Kensinger E. A., Sleep promotes lasting changes in selective memory for emotional scenes. Front. Integr. Nuerosci. 6, 108 (2012). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Payne J. D., et al. , Napping and the selective consolidation of negative aspects of scenes. Emotion 15, 176–186 (2015). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Alger S. E., Kensinger E. A., Payne J. D., Preferential consolidation of emotionally salient information during a nap is preserved in middle age. Neurobiol. Aging 68, 34–47 (2018). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Cunningham T. J., et al. , Psychophysiological arousal at encoding leads to reduced reactivity but enhanced emotional memory following sleep. Neurobiol. Learn. Mem. 114, 155–164 (2014). [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Bennion K. A., Payne J. D., Kensinger E. A., The impact of napping on memory for future-relevant stimuli: Prioritization among multiple salience cues. Behav. Neurosci. 130, 281–289 (2016). [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Bennion K. A., Mickley Steinmetz K. R., Kensinger E. A., Payne J. D., Sleep and cortisol interact to support memory consolidation. Cereb. Cortex 25, 646–657 (2015). [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Bennion K. A., Payne J. D., Kensinger E. A., Residual effects of emotion are reflected in enhanced visual activity after sleep. Cogn. Affect. Behav. Neurosci. 17, 290–304 (2017). [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Chambers A. M., Payne J. D., The influence of sleep on the consolidation of positive emotional memories: Preliminary evidence. AIMS Neurosci. 1, 39–51 (2014). [ Google Scholar ]
- 31. Cunningham T. J., Chambers A. M., Payne J. D., Prospection and emotional memory: How expectation affects emotional memory formation following sleep and wake. Front. Psychol. 5, 862 (2014). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Cunningham T. J., et al. , Higher post-encoding cortisol benefits the selective consolidation of emotional aspects of memory. Neurobiol. Learn. Mem. 180, 107411 (2021). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Vargas I., Payne J. D., Muench A., Kuhlman K. R., Lopez-Duran N. L., Acute sleep deprivation and the selective consolidation of emotional memories. Learn. Mem. 26, 176–181 (2019). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Ford J. H., Addis D. R., Giovanello K. S., Differential effects of arousal in positive and negative autobiographical memories. Memory 20, 771–778 (2012). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Holland A. C., Kensinger E. A., Emotion and autobiographical memory. Phys. Life Rev. 7, 88–131 (2010). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Kensinger E. A., Garoff-Eaton R. J., Schacter D. L., Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. J. Mem. Lang. 56, 575–591 (2007). [ Google Scholar ]
- 37. Kim S. Y., Payne J. D., Neural correlates of sleep, stress, and selective memory consolidation. Curr. Opin. Behav. Sci. 33, 57–64 (2020). [ Google Scholar ]
- 38. Bennion K. A., Payne J. D., Kensinger E. A., Selective effects of sleep on emotional memory: What mechanisms are responsible? Transl. Issues Psychol. Sci. 1, 79–88 (2015). [ Google Scholar ]
- 39. Kensinger E. A., Remembering the details: Effects of emotion. Emot. Rev. 1, 99–113 (2009). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Cowan E. T., Schapiro A. C., Dunsmoor J. E., Murty V. P., Memory consolidation as an adaptive process. Psychon. Bull. Rev. 28, 1796–1810 (2021). [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Waring J. D., Kensinger E. A., Effects of emotional valence and arousal upon memory trade-offs with aging. Psychol. Aging 24, 412–422 (2009). [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Peterson R. A., On the use of college students in social science research: Insights from a second-order meta-analysis. J. Consum. Res. 28, 450–461 (2001). [ Google Scholar ]
- 43. Henrich J., Heine S. J., Norenzayan A., The weirdest people in the world? Behav. Brain Sci. 33, 61–83, discussion 83–135 (2010). [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Redline S., et al. , The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch. Intern. Med. 164, 406–418 (2004). [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Ohayon M. M., Carskadon M. A., Guilleminault C., Vitiello M. V., Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 27, 1255–1273 (2004). [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Djonlagic I., et al. , Macro and micro sleep architecture and cognitive performance in older adults. Nat. Hum. Behav. 5, 123–145 (2021). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Helfrich R. F., Mander B. A., Jagust W. J., Knight R. T., Walker M. P., Old brains come uncoupled in sleep: Slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron 97, 221–230.e4 (2018). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Mander B. A., Winer J. R., Walker M. P., Sleep and human aging. Neuron 94, 19–36 (2017). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Mander B. A., et al. , β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat. Neurosci. 18, 1051–1057 (2015). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Scullin M. K., Sleep, memory, and aging: The link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol. Aging 28, 105–114 (2013). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Leong R. L. F., Lo J. C., Chee M. W. L., Sleep-dependent prospective memory consolidation is impaired with aging. Sleep 44, zsab069 (2021). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Muehlroth B. E., Rasch B., Werkle-Bergner M., Episodic memory consolidation during sleep in healthy aging. Sleep Med. Rev. 52, 101304 (2020). [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Kwon Y., Scheibe S., Samanez-Larkin G. R., Tsai J. L., Carstensen L. L., Replicating the positivity effect in picture memory in Koreans: Evidence for cross-cultural generalizability. Psychol. Aging 24, 748–754 (2009). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Reed A. E., Chan L., Mikels J. A., Meta-analysis of the age-related positivity effect: Age differences in preferences for positive over negative information. Psychol. Aging 29, 1–15 (2014). [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Jones B. J., Schultz K. S., Adams S., Baran B., Spencer R. M. C., Emotional bias of sleep-dependent processing shifts from negative to positive with aging. Neurobiol. Aging 45, 178–189 (2016). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Lachman M. E., Mind the gap in the middle: A call to study midlife. Res. Hum. Dev. 12, 327–334 (2015). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Jones B. J., Mackay A., Mantua J., Schultz K. S., Spencer R. M. C., The role of sleep in emotional memory processing in middle age. Neurobiol. Learn. Mem. 155, 208–215 (2018). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Gaudreau H., Carrier J., Montplaisir J., Age-related modifications of NREM sleep EEG: From childhood to middle age. J. Sleep Res. 10, 165–172 (2001). [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Button K. S., et al. , Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376 (2013). [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Stanley T. D., Carter E. C., Doucouliagos H., What meta-analyses reveal about the replicability of psychological research. Psychol. Bull. 144, 1325–1346 (2018). [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Baran B., Pace-Schott E. F., Ericson C., Spencer R. M. C., Processing of emotional reactivity and emotional memory over sleep. J. Neurosci. 32, 1035–1042 (2012). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Loftus E. F., Loftus G. R., Messo J., Some facts about “weapon focus.”. Law Hum. Behav. 11, 55–62 (1987). [ Google Scholar ]
- 63. Kim S. Y., et al. , Interactive effects of stress reactivity and rapid eye movement sleep theta activity on emotional memory formation. Hippocampus 30, 829–841 (2019). [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. van Marle H. J. F., Hermans E. J., Qin S., Overeem S., Fernández G., The effect of exogenous cortisol during sleep on the behavioral and neural correlates of emotional memory consolidation in humans. Psychoneuroendocrinology 38, 1639–1649 (2013). [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Kensinger E. A., Corkin S., Two routes to emotional memory: Distinct neural processes for valence and arousal. Proc. Natl. Acad. Sci. U.S.A. 101, 3310–3315 (2004). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Payne J. D., Kensinger E. A., Sleep’s role in the consolidation of emotional episodic memories. Curr. Dir. Psychol. Sci. 19, 290–295 (2010). [ Google Scholar ]
- 67. Ellenbogen J. M., Payne J. D., Stickgold R., The role of sleep in declarative memory consolidation: Passive, permissive, active or none? Curr. Opin. Neurobiol. 16, 716–722 (2006). [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Payne J. D., Kensinger E. A., Stress, sleep, and the selective consolidation of emotional memories. Curr. Opin. Behav. Sci. 19, 36–43 (2018). [ Google Scholar ]
- 69. Wagner U., Hallschmid M., Rasch B., Born J., Brief sleep after learning keeps emotional memories alive for years. Biol. Psychiatry 60, 788–790 (2006). [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Dinges D. F., Powell J. W., Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput. 17, 652–655 (1985). [ Google Scholar ]
- 71. Wang S. Y., et al. , ‘Sleep-dependent’ memory consolidation? Brief periods of post-training rest and sleep provide an equivalent benefit for both declarative and procedural memory. Learn. Mem. 28, 195–203 (2021). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Basner M., Mollicone D., Dinges D. F., Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 69, 949–959 (2011). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Buysse D. J., Reynolds C. F. III, Monk T. H., Berman S. R., Kupfer D. J., The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989). [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Kanady J. C., Harvey A. G., Development and validation of the Sleep Inertia Questionnaire (SIQ) and assessment of sleep inertia in analogue and clinical depression. Cognit. Ther. Res. 39, 601–612 (2015). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. de Leeuw J. R., jsPsych: A JavaScript library for creating behavioral experiments in a Web browser. Behav. Res. Methods 47, 1–12 (2015). [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Sanders K. E. G., Denis D., Sleep preferentially consolidates negative aspects of human memory: Well-powered evidence from two large online experiments. Open Science Framework. https://osf.io/24q6m/ . Deposited 13 February 2022. [ DOI ] [ PMC free article ] [ PubMed ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data availability statement.
- View on publisher site
- PDF (937.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Memory and Sleep: How Sleep Cognition Can Change the Waking Mind for the Better
Affiliation.
- 1 Department of Psychology and Cognitive Neuroscience Program, Northwestern University, Evanston, Illinois 60208, USA; email: [email protected], [email protected], [email protected].
- PMID: 32946325
- PMCID: PMC7983127
- DOI: 10.1146/annurev-psych-010419-050815
The memories that we retain can serve many functions. They guide our future actions, form a scaffold for constructing the self, and continue to shape both the self and the way we perceive the world. Although most memories we acquire each day are forgotten, those integrated within the structure of multiple prior memories tend to endure. A rapidly growing body of research is steadily elucidating how the consolidation of memories depends on their reactivation during sleep. Processing memories during sleep not only helps counteract their weakening but also supports problem solving, creativity, and emotional regulation. Yet, sleep-based processing might become maladaptive, such as when worries are excessively revisited. Advances in research on memory and sleep can thus shed light on how this processing influences our waking life, which can further inspire the development of novel strategies for decreasing detrimental rumination-like activity during sleep and for promoting beneficial sleep cognition.
Keywords: consolidation; learning; sleep; targeted memory reactivation; well-being.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Research Support, U.S. Gov't, Non-P.H.S.
- Cognition / physiology*
- Memory / physiology*
- Mental Health*
- Mental Recall
- Sleep / physiology*
Grants and funding
- R01 NS112942/NS/NINDS NIH HHS/United States
- T32 HL007909/HL/NHLBI NIH HHS/United States
- T32 NS047987/NS/NINDS NIH HHS/United States
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Sleep Improves Memory: The Effect of Sleep on Long Term Memory in Early Adolescence
* E-mail: [email protected] (WEB); [email protected] (KTP)
Affiliation Department of Human Biology, Brown University, Providence, Rhode Island, United States of America
Affiliation Department of Psychiatry and Human Behavior, University of California Irvine, Irvine, California, United States of America
- Katya Trudeau Potkin,
- William E. Bunney Jr

- Published: August 7, 2012
- https://doi.org/10.1371/journal.pone.0042191
- Reader Comments
Sleep plays an important role in the consolidation of memory. This has been most clearly shown in adults for procedural memory (i.e. skills and procedures) and declarative memory (e.g. recall of facts). The effects of sleep and memory are relatively unstudied in adolescents. Declarative memory is important in school performance and consequent social functioning in adolescents. This is the first study to specifically examine the effects of normal sleep on auditory declarative memory in an early adolescent sample. Given that the majority of adolescents do not obtain the recommended amount of sleep, it is critical to study the cognitive effects of normal sleep. Forty male and female normal, healthy adolescents between the ages of ten and fourteen years old were randomly assigned to sleep and no sleep conditions. Subjects were trained on a paired-associate declarative memory task and a control working memory task at 9am, and tested at night (12 hours later) without sleep. The same number of subjects was trained at 9pm and tested 9am following sleep. An increase of 20.6% in declarative memory, as measured by the number correct in a paired-associate test, following sleep was observed compared to the group which was tested at the same time interval without sleep (p<0.03). The performance on the control working memory task that involved encoding and memoranda manipulation was not affected by time of day or relationship to sleep. Declarative memory is significantly improved by sleep in a sample of normal adolescents.
Citation: Potkin KT, Bunney WE Jr (2012) Sleep Improves Memory: The Effect of Sleep on Long Term Memory in Early Adolescence. PLoS ONE 7(8): e42191. https://doi.org/10.1371/journal.pone.0042191
Editor: Antonio Verdejo García, University of Granada, Spain
Received: February 10, 2012; Accepted: July 4, 2012; Published: August 7, 2012
Copyright: © Potkin, Bunney Jr. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The authors have no support or funding to report.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Several studies primarily in adults have shown that sleep improves procedural memory, i.e. skills and procedures [1] , [2] as well as declarative memory [3] . REM and slow-wave sleep (SWS) have been implicated in memory consolidation [3] – [5] . Lack of REM sleep is associated with poor recall of visual location [6] . Decline in declarative memory consolidation is correlated with a decline in slow-wave sleep [7] . Spencer et al. observed similar initial procedural learning in older and younger adults; however, the older adults’ performance did not improve following sleep, suggesting that sleep dependent memory consolidation decreases with age [8] . This may reflect the disturbed sleep and disrupted SWS in the elderly [3] , [8] , [9] . Slow wave sleep increases until shortly before puberty and then shows a prominent drop across adolescence, decreasing by more than 60% between ages 10 and 20 years [10] . It is critical to understand the cognitive effects of normal sleep in order to understand the consequences of disrupted sleep. This is important since the majority of adolescents do not obtain the recommended amount of sleep and that disrupted sleep is a key symptom in most adolescent psychiatric and developmental disorders [11] .
Backhaus et al. studied twenty-seven children with an average age of 10.1 years (range of nine to twelve), on a learned word pairs list, employing a within subject design and two post-learning assessments. They found that declarative memory was significantly increased immediately after an interval of sleep, as well as with delayed post-learning sleep [12] . As the authors had noted, no control task was administered to determine if circadian confounds were responsible for this increase in recall post sleep. Our study addressed this limitation by administering a control task and evaluating the effect of sleep on auditory declarative memory consolidation in early adolescence. Visual declarative memory has been reported to be enhanced following sleep in children; however, auditory declarative memory has not been previously studied [13] .
Participants
Twenty female and twenty male adolescents, between the ages of 10 and 14, were recruited in a public middle school. The study was considered exempt by the institutional review board because it involved the use of educational tests without personal subject identifiers. In accord with the principals of the Declaration of Helsinki, subjects were asked to participate in a school class project and only told that they would be tested two times for about 15 minutes each time. Subjects with academic failure or accelerated academic performance or sleep problems were not included. The subjects agreeing to participate were grouped by sex and assigned to sleep or no sleep conditions with a separate randomization table for each group, to ensure a balanced design.
Subjects were tested in their homes in a quiet room without distractions for the duration of the learning and testing. The testing sessions were conducted during weekends or during school break. All subjects were given the paired-associate test, one of the standard tests of declarative memory [14] , which consisted of repeating semantically related and unrelated pairs of words (e.g. tree/leaf; lamp/shoe), in a standardized manner. After each word pair was presented out loud, the subject repeated the pair out loud to ensure registration of the paired associate. The list of the same 10 pairs was administered three times in immediate succession. Subjects assigned to the sleep condition learned the paired associates at 9∶00pm (±30 minutes), and were tested for cued recall twelve hours later, after a night of sleep. The no-sleep group received the same paired-associate presentation at 9∶00am (±30 minutes) and was tested for recall twelve hours later, with no intervening sleep or naps. The control working memory task, letter-number, was given just prior to learning the paired-associate words and again just prior to being tested on the paired-associate words. The letter-number test was administered to control for possible circadian confounds and to control for attention and encoding. The letter-number control task (LN, immediate recall and reordering of letters and numbers) is a subtest of the WAIS-III (Wechsler Adult Intelligence Scale) and WMS-III (Wechsler memory Scale), the most widely used intelligence and memory scales. An increasing long series of mixed letters and numbers is read to the subject and the subject then orders the numbers and letters in ascending order, e.g. b3a1 is read and subject correctly responds with 13ab. The letters and numbers must be encoded and then manipulated to get the correct answer. Two versions of the letter-number task were used in random order. The number correct was scored for the paired-associate and the letter-number tests. The memory scores were transformed into Z scores to determine if outliers were present; an exclusionary Z score of ±2.57 was applied (1% of the normal distribution). Between group comparisons were calculated by students t-test (2 tailed) after testing for equal variances by Levene’s test, and ANCOVA as necessary. Within subject comparisons were calculated by paired t-test.
Subjects were instructed to eat their usual meals approximately one hour before learning the paired-associates and one hour before being tested on the paired-associates. Subjects were instructed to get a good night’s sleep. All the subjects included reported having had typical night of sleep and rated the quality of the sleep as good to very good prior to the testing.
The sleep group’s mean age was 12.9 compared to 12.4 for the non-sleep group (t = (1.52), df (1,38), p = 0.14). (See Table 1 for demographic characteristics and performance scores). There was no statistically significant sex difference in performance for either task.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0042191.t001
Three outliers were identified and removed; one high scoring subject assigned to the sleep and two lower scoring subjects assigned to no sleep. After removing outliers, 19 sleep subjects and 18 no- sleep subjects remained. The Levene’s Test showed equality of variances for all comparisons. The number correct on the letter-number control task at initial testing was 6.58 for the sleep group and 6.06 for the no-sleep group, (t = (1.54), df (1,35), p = 0.13). The letter-number correct score on the second administration was 6.26 and 6.33, respectively, (t = (-.16), df (1,35), p = 0.88), ( Figure 1 ). There was also no statistically significant difference in performance for either group on letter-number task between the first and second administration (paired t test, p = 0.32 for sleep group and 0.45 for no-sleep group).
A histogram of mean number correct (± SD) for the Paired-Associate Test (PA) and Letter Number Test (Letter #), with (n = 19) (outliers removed) and without sleep (n = 18).
https://doi.org/10.1371/journal.pone.0042191.g001
An increase of 20.6% in long-term memory ( Figure 1 ) was found as measured by the number correct in the paired-associate test following sleep, compared to the group which was tested at the same time interval, but without sleep (p<0.029). When the three outliers are included, the number correct for recall of the paired-associates was statistically greater for the sleep group (7.5) compared to the no sleep group (5.9, t = (2.76), df (1,37), p<0.009), a 32.7% increase.
The paired-associate test is one of the standard tests of declarative memory and has been previously used to study declarative memory and the effects of sleep on declarative memory in adults and children [3] . All subjects were evaluated at the same two times of day, approximately 9 AM and 9 PM, using standardized conditions. Performance on the paired-associate test was significantly affected by sleep in our adolescent sample. In contrast, working memory performance as measured by the letter-number test, a standard subtest of the WAIS-III and WMS-III was not affected by time of day or in relation to sleep. Correct performance on the letter-number working memory task (LN) requires that the letters and numbers presented to the subject must be encoded and then correctly manipulated. We had 80% power to detect a standardized difference of.76 correct (∼11% change) or greater, following sleep. A small difference in working memory performance (<11%), however, may exist that could not be detected with our sample size.
The equal performance at both sessions and between groups on the LN supports the view that equal registration and encoding of the memoranda was comparable at both time points and between groups. Performance on the working memory control task did not change with the second session for either group, suggesting that the time of day had no effect on performance on the working memory control task. Consequently, the observed difference in paired-associate performance, i.e. consolidation of working memory, is most likely related to sleep itself and not any differences in encoding. Memory consolidation has been reported to be affected by sleep [1] , [2] , [8] , [9] . Both REM and slow-wave sleep have been associated with improved memory [3] – [5] . Slow wave sleep particularly enhances declarative memory. 7 .
Our results are consistent with Gais et al.’s study of young males (mean age 17.4) showing that enhanced declarative memory was related to periods of sleep, and not to time of day effects [15] . Naps improve declarative memory regardless of time of nap [16] and closely resembled memory improvement after an eight-hour night of sleep [17] . In reviewing the timing of sleep and circadian rhythms, Diekelmann et al. conclude that sleep promotes memory consolidation independently of the time of day in which it occurs [3] . Voderholzer et al. studying 14–16 year old adolescents showed that several nights of sleep restriction did not impact memory consolidation nor performance in a working memory task, when two recovery night of sleep were provided, an effect they ascribed to a compensatory enhancement of SWS [18] . The paired-associate test begins as a working memory task and after a period of time with consolidation becomes a declarative memory task. Correct performance on the letter-number test and the paired-associate tests are dependent upon encoding the memoranda.
A limitation of this study is that we did not test for encoding strength by immediate recall after the administration of the paired-associate test. The letter-number test requires attention and encoding. An element of immediate recall is to prove that the subject was attending. This was assured by having the subjects read the words (similar to other learning tests like the CERAD and ADAS-COG) and supported by the finding of the performance on the letter-number test. It is likely that if immediate recall following each presentation was obtained, higher accuracy rates would have been observed. Recent studies have demonstrated that salience increases declarative memory performance [13] , [19] . Nevertheless, our data demonstrate that sleep improves memory consolidation even in conditions where encoding has not been reinforced.
Neither time of day or sleep affected the performance on the letter-number test suggesting that the material was being learned and encoded. There is no evidence that memory consolidation depends on time of day independent of sleep. The lack of interference during sleep has been considered as a possible cause of the beneficial effects of sleep on declarative memory, i.e. there are no daytime demands to interfere with memory consolidation. Our design tested subjects on non-school days, thus mitigating the effects of interference of memory consolidation during the day by learning competition and other demands of a normal school day. Gais et al. controlled for waking associated interference and found no effect of interference on memory [15] . In a review of controversy regarding whether absence of interference accounts for memory improvement during sleep, Ellenbogen at al. point out “although sleep might passively protect declarative memories from interference, consolidation must also occur during sleep for the memories to become resistant to interference the following day”. Based on their review of related animal and human studies, they point out that “hippocampus-dependent memories are reactivated during sleep, and that this reactivation leads to strengthened memory traces”, finally concluding “that specific, sleep-dependent, neurobiological processes directly lead to the consolidation of declarative memories” [1] . Diekelmann et al. hypothesized that both encoding and sleep-dependent consolidation during sleep involve prefrontal-hippocampal circuitry [3] .
Children have high amounts of slow wave sleep and sleep in general. Sleep has been shown to improve declarative and procedural memory in children and older age groups. Subjects were asked about their sleep and confirmed that they had a typical night sleep, consisting of 8–10 hours of sleep, average for adolescents [20] . We did not, however, specifically measure sleep. Lack of sleep can result in poor cognitive performance, which was not observed in our sample, and is consistent with the subjects’ report of a good night sleep and that poor sleepers were excluded from the sample.
A cross-over design would have provided additional confirmation at the individual subject level in contrast to our parallel group design. Our study was limited as the sample was opportune, from a California middle school, and was not epidemiologically based. No subjects approached declined to participate. No accelerated or failing students were included, although this was not a strict exclusion criterion. There were 3% African-American, 5% Asian, and 92% Caucasian. The sample population reflected the general school population in this geographic area, although Asians were underrepresented (12.8%).
Our sample size was relatively small and limited to early adolescence, ages 10–14, although twice the sample of Prehn-Kristensen et al. who found 10 to 13 year olds improved visual memory following sleep, especially to emotional pictures [13] . The 10–14 age group was deliberately chosen because of the importance of declarative memory on adolescent school performance and related social functioning [21] . Marked changes in sleep and sleep architecture are a defining feature of adolescence [22] . Disorders of adolescence frequently disrupt sleep. Twenty-five to forty percent of adolescents have sleep disorders that can have an important effect on daytime school and consequent social functioning [23] . Sleep disorders are even more prevalent in adolescents with psychiatric disorders and developmental disabilities [24] . It is important to have data on the effects of normal sleep on declarative memory in normal adolescents to better understand the consequences of lack of sleep and abnormal sleep patterns.
Given the importance of adolescent memory on school performance and consequent social functioning, a fuller understanding of the effects of sleep on memory consolidation is needed. Other studies are needed to investigate the specific effects of sleep on other types of memory, such as visual, procedural, and emotional. Understanding the role of normal sleep on memory consolidation in adolescence is critical in identifying the consequences of disrupted sleep in adolescent disorders and their treatment.
Author Contributions
Conceived and designed the experiments: KTP WEB. Performed the experiments: KTP. Analyzed the data: KTP WEB. Contributed reagents/materials/analysis tools: KTP WEB. Wrote the paper: KTP WEB.
- View Article
- Google Scholar

IMAGES
COMMENTS
Further investigation of sleep-related memory consolidation mechanisms could thus lead to new insights into sleep that apply whether an individual has clinical symptoms or not. HARNESSING SLEEP TO IMPROVE PSYCHOLOGICAL WELL-BEING. The cumulative research using TMR shows that sleep-based consolidation is modifiable.
Dec 21, 2020 · Recent research has led scientists to hypothesize that Stage 3 (deep non-Rapid Eye Movement sleep, or Slow Wave Sleep) may be especially important for the improvement of memory retention and recall [2]. How does sleep improve long-term memory? Scientists hypothesize that sleep also plays a major role in forming long-term memories.
The contribution of sleep to improvements in working memory scanning speed: A study of prolonged sleep restriction. Biol Psychol. 2006;72:208–12. doi: 10.1016/j.biopsycho.2005.11.002. [PMC free article] [Google Scholar] Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation.
6 days ago · Sleep is essential for cognitive development; it helps the brain integrate new and existing knowledge, making learning long-lasting rather than transient 8,9,10.Building on previous research ...
Research suggests that sleep deprivation both before and after encoding has a detrimental effect on memory for newly learned material. However, there is as yet no quantitative analyses of the size of these effects. We conducted two meta-analyses of studies published between 1970 and 2020 that investigated effects of total, acute sleep deprivation on memory (i.e., at least one full night of ...
This study found sleep improved next-day memory retention in young adults but not older ones, with the age-related decline being particularly pronounced for declarative memory tasks. The research points to a reduction in SWS as a contributing factor, which is critical for declarative memory and known to diminish with age.
Nov 11, 2022 · Despite decades of research showing that sleep strengthens memory above and beyond wakefulness (7, 8), sleep’s role in memory remains contested . Moreover, studies from different laboratories have shown that sleep prioritizes the consolidation of emotional over neutral memories, and to a larger degree than occurs across wake ( 9 – 13 ).
Research indicates that sleep promotes various cognitive functions, such as decision-making, language, categorization, and memory. Of these, most work has focused on the influence of sleep on memory, with ample work showing that sleep enhances memory consolidation, a process that stores new memories in the brain over time. Recent psychological ...
Jan 4, 2021 · A rapidly growing body of research is steadily elucidating how the consolidation of memories depends on their reactivation during sleep. Processing memories during sleep not only helps counteract their weakening but also supports problem solving, creativity, and emotional regulation.
Aug 7, 2012 · Sleep plays an important role in the consolidation of memory. This has been most clearly shown in adults for procedural memory (i.e. skills and procedures) and declarative memory (e.g. recall of facts). The effects of sleep and memory are relatively unstudied in adolescents. Declarative memory is important in school performance and consequent social functioning in adolescents. This is the ...