Science Project Ideas

Gummy Bear Experiment
The gummy bear experiment is a fun activity that teaches the basic concept of osmosis to the little ones in an easy manner. They will also be thrilled at the idea that their favorite gummy bears could teach them a lesson or two in science.
Gummy Bear Science Project Instructions
Hypothesis for growing/shrinking gummy bears.
When a gummy bear is placed in a hypotonic solution (e.g. water) then it will increase in mass and volume. On the other hand, its mass and volume decrease when placed in a hypertonic solution (e.g. salt water). Hypertonic refers to a higher concentration of solutes and hypotonic is just the reverse.
Things Needed
- Physical balance or digital weighing machine
- Sieve, plastic fork or screen
- Graph paper
- Measure the dimensions (length, breadth and height) of the bear with a scale. Measure its mass with the balance.
- Fill the bowl with water.
- Completely immerse the gummy bear in the water.
- Let the bowl sit overnight in a place away from direct sunlight.
- Next day, lift the bear from the water with a plastic fork, sieve or screen.
- Record the dimensions and mass of the bear again.
- Do the same observation each day for a couple of days more.
- Plot a graph with the time in hours along the X-axis (the dependent variable) and the mass or weight of the gummy bear along the Y-axis (independent variable). Check the nature of the graph.
Things You Can Try
- Set up a series of bowls on the table and fill them with different solutions like that of baking soda, vinegar, salt, distilled water, etc. Make similar observations as above for each one of them. Compare your results.
- Also, check if the taste and/or color of the bears have changed.
Gummy Bear Osmosis Video
How does it work.
The ingredients of gummy bears are sugar, water, and gelatin, with little water content. Due to the process of osmosis, i.e., the movement of water molecules through a selectively permeable membrane from an area of high concentration to that of a lower concentration, the bear starts to grow. However, it doesn’t get dissolved as the gelatin is insoluble in water.
On trying out the different ideas, you will find that the degree of expansion of the candy depends on the liquid on which it is kept. However, vinegar, which is actually an acid, can dissolve the candy .
This simple trick can prove to be a cool science fair idea. The kids will be enthralled to display their knowledge to the audience in a fun way.
References:
https://biozone.weebly.com/uploads/2/7/4/2/274298/gummy_bear_osmosis.pdf
https://tinkerlab.com/incredible-growing-gummy-bears/
https://www.childrensmuseum.org/blog/saturday-science-growing-gummy-bears
https://www.homeschool.com/blog/index.php/2014/04/homeschool-science-gummy-bear-osmosis/
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

Home » Free Homeschooling » Gummy Bear Osmosis
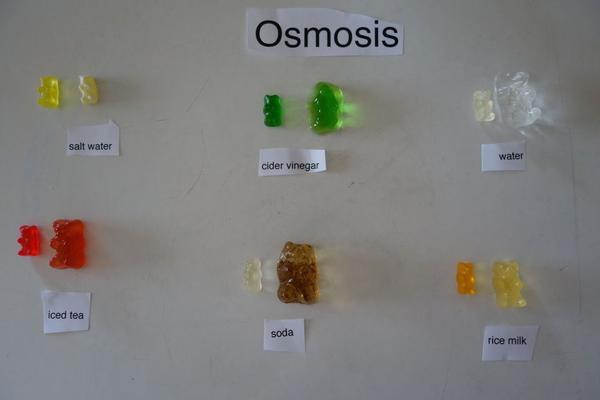
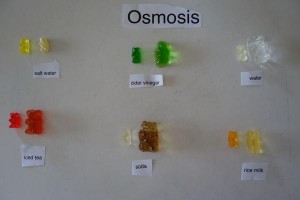
Gummy Bear Osmosis

Check out our FUN Gummy Bear Osmosis experiment!
This is a simple and fun experiment for children 12 and under (and their moms).
A quick safety note:
Don’t let your kids eat the gummy bears after they’ve soaked in the various solutions. The ones in baking soda and vinegar will taste awful, and they will all contain bacteria as your kids will be handling them and then putting them back in the fluids.
AND, if you have a cat like we do, the cat might lick from the containers and/or drop a hair or two among the various solutions.
BTW – because of our cat, I did talk about the importance of strict scientific procedures and how data can be contaminated. My kids know that our experiment was more of a fun experiment – and that some contamination undoubtedly occurred.
Just a thought – you might want to buy TWO bags of gummy bears – one for the experiment and one for nibbling.
_____________________________________________________________________
Some educational info before you start (so you can explain the science to your kids):
Most sugary candy dissolves in water. In fact, you might want to try this experiment first, so your kids understand that this is true.
Gummy bears are an exception – they don’t dissolve in water . This is because they’re made with gelatin . When gummy bears are made, gelatin and water are heated and mixed (like when you make gelatin at home). As the mixture cools, water leaves the candy and the candy hardens and becomes gummy/chewy.
When you put a gummy bear in water, it is a solute , and the water molecules are a solvent. Since the gummy bear does not contain water (remember, the water was removed when the gummy bear was made), water now moves into the bear by the process of osmosis . Osmosis is the process whereby water moves from a greater concentration of water to a lower concentration of water (from a container of water to the candy bear).
Also, gummy bears have a semi-permeable membrane – their surface has holes in it and these holes allow small, non-charged particles like water in, but don’t let larger particles (like sugar) out.
At the beginning of the experiment, there is less water and more gelatin inside each gummy bear. As time passes, this changes, as the gelatin makes the gummy bear act like a sponge, absorbing water rather than being dissolved in it (like other candies).
Try the Gummy Bear Osmosis experiment and see what happens!
_______________________________________________________________
This Gummy Bear Osmosis experiment takes less than an hour to set up, but the actual experiment runs for 48 hours.
Materials You Need for the Gummy Bear Osmosis Experiment
- Gummy Bears
- A glass container for each liquid/solution
- One tablespoon of salt
- A tablespoon of sugar
- One tablespoon of baking soda
- Kitchen scale
- Paper towels
- Clock or timer
- Gummy Bear Experiment Sheet (included at the end of this post, although the spacing is slightly different)
- Gummy Bear Scientific Data Table (included at the bottom of this post)
Instructions for the Gummy Bear Osmosis Experiment
- Label each glass with its contents: water, salt water, sugar water, etc.
- Fill the glass labeled water with one-half cup plain water.
- Fill the glass labeled salt water with one-half cup water. Thoroughly mix in one tablespoon of salt (make sure all the salt is dissolved).
- Fill the glass labeled sugar water with one-half cup water. Thoroughly mix in one tablespoon of sugar (again, make sure all the sugar is dissolved).
- Fill the glass labeled baking soda water with one-half cup water and thoroughly mix in one tablespoon of baking soda.
- Fill the other containers with their respective liquids.
- Select a gummy bear for each glass.
- Measure the length, height and width of each gummy bear, weigh each gummy bear and write this info on the Gummy Bear Scientific Data Table. If your scale isn’t able to weigh just one gummy bear you can still do this experiment. Visually, your kids will be able to see the difference in the bears pre- and post-experiment.
- Check – and write down the time.
- Now, add a gummy bear to each glass.
- Wait 12 hours.
- Remove the gummy bears from their respective glasses.
- Let your kids ooh and ahh and talk about the differences in the bears.
- Now, measure and if you can,weigh each bear. Use the Gummy Bear Scientific Data Table to write down your results.
- Put the gummy bears back in their solutions.
- Check back after 24 hours and again measure and weigh each gummy bear. Again, give your kids plenty of time to talk about the results.
- Again, put the gummy bears back in their glasses.
- Check back after 48 hours, measure and weigh each gummy bear. Use the Gummy Bear Scientific Data Table to write down your results.
What did you discover? Did your gummy bears GROW – by A LOT!?! Which gummy bears grew the most, without losing shape?
Explain scientifically what happened., check out our experiment below., this is the beginning of it..

(our cat lost interest quickly)
Gummy Bear Osmosis Experiment – After 12 hours

Gummy Bear Osmosis Experiment – After 24 hours

Our water gummy bear has lost part of its leg! And I can clearly see gummy particles in some of the water solutions.
But our vinegar gummy bear isn’t a blob yet, and I’ve heard that’s what happens. I’m getting a little worried – did we do something wrong?
Gummy Bear Osmosis Experiment – After 48 hours

A lot of the gummy bears are falling apart after 48 hours! I originally planned to check again after 72 hours – but they just won’t make it! The vinegar gummy is a blob (yeah!), and the water and soda gummy bears are literally falling apart. This gummy bear osmosis experiment was so much fun for the kids!
From http://mirada.oursciencefair.com/SchoolHome.aspx

I’ve included the forms I used below – in case you’d like to use them ____________________________________________________________________________
GUMMY BEAR SCIENCE EXPERIMENT
Gummy bear osmosis.

TAP WATER
DISTILLED WATER
SALT WATER
BAKING SODA WATER
SUGAR WATER
SODA
_____________________________________________________________
Scientific Data Table

You may also like

Free Homeschool Curriculum

Coronavirus Home Schooling – How to Homeschool...

Homeschool-Freebies

Homemade Ice Cream – YUM

Pumpkin Cloud Dough – DIY Fun

Dissolving & Bouncing Egg Experiment
😮 The 50 greatest innovations of 2024: From Zildjian to NASA 😮
- svg]:fill-accent-900 [&>svg]:stroke-accent-900">
Stay-at-home science project: Enlarge gummy bears to reveal the secrets of osmosis
By Rachel Feltman
Posted on May 4, 2020
Welcome to PopSci’s at-home science projects series . On weekdays at noon, we’ll be posting new projects that use ingredients you can buy at the grocery store. Show us how it went by tagging your project on social media using #popsciprojects.
Gummy bears are delicious. That’s not up for debate (though you’re welcome to eat a few to prove the hypothesis). But they’re also the perfect critters to help demonstrate a process that makes life as we know it possible: Osmosis.
Believe it or not, osmosis also happens when you drop gummy bears into water, revealing the most basic inner workings of your body’s cells. Just add water and a spoonful of salt to see it happen before your eyes.
- Time: 5 minutes of prep, and 3 to 9 hours of waiting
- Difficulty: easy
What you’ll need
- Gummy bears, preferably dark in color
- Three small bowls
- 2 tablespoons of salt
- (Optional) Ruler
- (Optional) Kitchen scale
Instructions
1. Fill two of your bowls with cool water. Room temperature is fine, but keep away from hot water—it’ll melt your gummy bears.
2. Add the salt to the first bowl. The second should just contain water.
3. Plop one gummy bear into each bowl. Make sure they’re fully submerged. Leave the bowls somewhere they won’t be disturbed. Gummy bears are tempting—even when they’re salty and soggy!
- Note : We tried different types of gummies (including the sour kind coated with mouth-puckering crystals), and we came to the conclusion that the ideal gummy bears for this experiment are dark in color and chewy instead of soft—just the classic gummy bear . A darker color will not dilute so much as to turn the gummy bear totally clear as it absorbs water, and the thicker gelatin mixture will make the candy less likely to fall apart when you take it out for observation. Also, stay away from sour gummies and those with unusual flavor additives, since they are less likely to yield the intended results.
4. Set aside a third gummy as your experimental control. We recommend you do this before you even think about eating the rest of your gummy bears. It’d be tragic to suddenly realize you ate them all and you no longer have a control for your experiment. Keep it dry.
5. Wait for three hours.
6. Check back in on your waterlogged candies. You can scoop them out with a spoon and observe them on a paper towel if you so choose, but be sure to return them to their proper bowls. Take note of how the gummies have changed—write down your observations so you can contrast them with the end results. You can check in again after the next three hours.
7. (Optional) Take some measurements. If your little experimenters need more of a challenge, you can have them measure the bears with rulers and/or kitchen scales, and calculate just how much size and mass the bears have lost or gained.
8. Wait another six hours. The full transformation should be complete around hour nine.
9. Retrieve your bears from their bowls. Use a small spoon and line them up on a plate or paper towel to see how much they’ve changed. The gummy in plain water should be much larger than the unsoaked candy, while the salted water should have kept its bear roughly the same size—unless it’s caused it to shrink. More on that later.
10. Fill a third bowl with cool water and a tablespoon of salt. Place the expanded, waterlogged gummy bear into it and observe it every few hours. It should get noticeably smaller as it soaks.
How it works
Osmosis is the movement of water through a semipermeable membrane—that is, a material with holes large enough to let some things in, but small enough to keep others out. In this process, water moves through the membrane without force or energy, to make water concentration versus other molecules roughly the same on either side.
This process is important in keeping us alive. The outer membrane of our cells is semipermeable and allows small molecules like water and oxygen to pass through while keeping all the cell’s organelles protected and in place. When it’s time to eliminate waste, the cell will start pushing the toxic molecules out, while absorbing water from our blood through osmosis. Once the cell has balanced its water concentration to the one outside of it, it will stop taking in more liquid, thus preventing the cell from bursting.
Gummy bears are made of gelatin and sugar, and the proteins that make up gelatin are very similar to the outer membranes of our cells. Just like them, the gummy bear’s gelatin “skin” will allow water and other small molecules to pass through while keeping larger ones contained—in this case, those larger molecules are the sugar that make gummy bears taste so good.
When you place a gummy bear into water, the sugar molecules will try to spread out and disperse evenly through the water bowl. But the gelatin membrane won’t let them out. That sugar also makes the gummy have a relatively low concentration of water compared to the liquid around it. Osmosis seeks to correct this imbalance, so water will keep pushing into the gummy and through the membrane until the concentration is the same on either side of the gelatin. This means your gummy is going to absorb lots and lots of water.
In a solution of water and salt, the bowl and the bear have similar water concentrations, so the candy may stay about the same size or even shrink, if the water is salty enough. Just as the sugar in the bear lowers its water concentration, the salt in the bowl means a lower ratio of water to other molecules. As a response, the bear may push out water in order to dilute the liquid inside the bowl. If you place the water-swelled bear into a salt solution, those extra water molecules will leave the bear to lower the salt concentration in the bowl.
If you have time (and gummies) to spare, you can elaborate on this experiment by testing different salt concentrations. You can line up several bowls with increasing quantities of salt in the same amount of water. The more salt you add, the more your candy should shrink.

Win the Holidays with PopSci's Gift Guides
Shopping for, well, anyone? The PopSci team’s holiday gift recommendations mean you’ll never need to buy another last-minute gift card.

Growing Gummy Bear Science Experiment
This gummy bear osmosis activity is great for showing children the scientific method. Teach your kids the simple steps with a mess-free gummy bear science experiment.

How does a baby gummy bear grow up to be a big gummy bear?
It seems like a riddle that cannot be solved. After all, gummy bears are candies, so how can they grow?
The answer is … through science! Or specifically, through osmosis.
Check out how osmosis can grow your gummy bears (and also shrink!) through the simple science experiment below!
The Gummy Bear Osmosis Experiment
I am including all the different liquids and solutions we tested below. You do not have to do all of them but I suggest selecting at least a couple to try out besides water.
Materials:
- Gummy bears
- Baking Soda
- Optional: Gummy Bear Experiment Worksheet (I recommend laminating it so it doesn’t get wet)
Instructions:
- Using paper or post-its, label each container with its contents: water, salt water, sugar water, vinegar, etc.
- Fill the container labeled water with ½ cup of tap water.
- Heat 1.5 cups of water in the microwave or on the stovetop.
- Pour ½ cup of hot water into the container labeled salt water. Add salt a little bit at a time, stirring every time. When the water is saturated with salt (no more salt is dissolving), stop and leave the solution to cool (you can put it in the fridge to make it cool faster). If you put gummy bears in warm or hot water, they will dissolve.
- Repeat step #4 with sugar instead of salt.
- Pour ½ cup of hot water in the container labeled baking soda and stir 1 tablespoon of baking soda.
- Fill the container labeled vinegar with ½ cup of vinegar.
- Fill the container labeled milk with ½ cup of milk.
- After all the liquids have reached room temperature, put a gummy bear in each container.
- Make predictions on what is going to happen to the gummy bears in the various liquids. Will they get bigger, smaller, or stay the same? You can record your predictions on the Gummy Bear Experiment Worksheet to keep track.
- Leave the gummy bears in the liquids for 12 hours. I did put the milk container in the fridge overnight because I didn’t want the milk to spoil, but that could have stunted the growth of the gummy bear by slowing down the rate of osmosis (need to experiment more to make that conclusion).
- Carefully take out the gummy bears from their respective containers and place them on a plate, or the Gummy Bear Experiment Worksheet if you are using it.
- Place a control gummy bear (one that has not been submerged in any liquid) next to each of the gummy bears you removed from the containers.
- Discuss with your child the difference in sizes. Did your child predict correctly what was going to happen to the gummy bears?

The Science Behind Growing Gummy Bears
The gummy bears are made up of water, sugar, and gelatin. The gelatin allows the gummy bears to grow in liquid instead of dissolves like other candies, as we observed in the melting Skittles or floating “m” M&Ms experiments.
The gelatin also acts like a semi-permeable membrane that allows water to enter the gummy bears.
Due to osmosis, water wants to either enter or exit the gummy bear to equalize the concentrations of each side of the membrane. For example, when the gummy bear is in plain water, there is more sugar inside the gummy bear. Therefore, water diffuses, or moves into, the gummy bear to decrease the amount of sugar versus water.
As water moves into the gummy bear, the gummy bear starts to grow. Eventually, the sugar concentration inside the gummy bear is the same as the sugar concentration inside the liquid, and osmosis stops.
If you truly saturated your salt or sugar solutions, your gummy bear should shrink. Water should have moved out of the gummy bear since there is more salt or sugar in the liquid. However, if your gummy bears grew (like ours did), that means the gummy bears still had more salt and water than the amount we dissolved in the water.
In vinegar, the gummy bear should have grown. However, since we left it in the vinegar overnight, the acid in the vinegar broke down the gelatin. As a result, our gummy bear dissolved and completely disappeared!
Check out the bouncy egg experiment to conduct another fun osmosis experiment!
Final Thoughts on the Gummy Bear Experiment
The gummy bear experiment takes a little time to set up, but the process is very simple. Your kid will have fun guessing what is going to happen to the gummy bears in the various solutions.
You can test out what will happen to the gummy bear in other liquids. You can try juice, sports drink, or even apple cider vinegar.
For older kids, you can also have them weigh and measure the gummy bears to compare the weight and size differences.

About The Author
5 thoughts on “growing gummy bear science experiment”.
wow nice experiment
I think this is a great what ever you call it but i think i am going to do this for my project this year!:)))))) LOL
haha it’s super fun!
I’m doing this for my science fair project! Thank you for the help. 🙂
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Get Your ALL ACCESS Shop Pass here →

Gummy Bear Osmosis Experiment
Learn about the process of osmosis when you try this easy gummy bear osmosis experiment with the kids. Watch your gummy bears grow as you investigate what liquid makes them grow the biggest. We are always on the hunt for simple science experiments and this one is just super fun and easy!

Explore Science With Gummy Bears
A fun gummy bear experiment all in the name of science and learning! There are so many simple science experiments that are quick and easy to set up for young children. Older children can easily add data collection, graphing and charts to turn this fun edible science experiment into more of a challenge!
Grab a bag of gummy bears or alternatively, you can make your own homemade gummy bears with our easy 3 ingredient gummy bear recipe .
Then head into the kitchen to grab your supplies and let’s find out what happens when you add gummy bears to different liquids. Watch your gummy bears as you investigate what makes gummy bears grow the biggest.
LOOK: 15 Amazing Candy Science Experiments
Set Up A Gummy Bear Osmosis Lab
Let’s find out what liquid makes gummy bears grow the biggest! Remember, the dependent variable is the size of the gummy bears and the independent variable is the liquid you use. Learn more about variables in science.
- Gummy bears
- baking soda
- ruler or measuring scale
- optional – stopwatch
TIP: Extend the experiment by using additional liquids such as juice, vinegar, oil, milk, baking soda mixed with water etc.

Instructions:
STEP 1. Carefully measure and pour the same amount of water into 3 cups. Add the same amount of distilled water to another cup if using. Pour the same amount of vinegar into another cup.
STEP 2. Add sugar to one cup of water, baking soda and salt in another. Mix well.

STEP 3. Weigh and/or measure each gummy bear beforehand. Use the printable worksheet above to record your measurements.
STEP 4. Add a gummy bear to each cup.

STEP 5. Then set the cups aside and wait to observe what will happen. Check them again after 6 hours, 12 hours and 24 hours.
TIP: This gummy bear experiment takes at least 12 hours to work!

STEP 6. Remove your gummy bear from the liquid and carefully measure and/or weigh each one. What liquid made the gummy bears grow the biggest? Why was that?

Free Printable Gummy Bear Lab Worksheet
Use the free gummy bear data sheet below to track your results! It’s perfect for older kids to add to a science notebook.

How Does Osmosis Occur In Gummy Bears?
The process of moving water across a semi-permeable membrane from a low concentrated solution to a high concentrated solution is called osmosis . A semi-permeable membrane is a thin sheet of tissue or layer of cells acting as a wall that allows only some molecules like water molecules to pass through.
The main ingredients in gummy bears are gelatin, sugar and flavoring. The semi-permeable membrane in gummy bears is the gelatin.
CHECK OUT: How To Make Slime With Gelatin
It is the gelatin that also stops the gummy bears from dissolving in liquids, other than an acidic solution such as vinegar.
When you place gummy bears in water, the water moves into them through osmosis since gummy bears don’t contain water. The water is moving from a low concentration solution to a high concentration solution.
Learn more about osmosis with our potato osmosis lab.
Using The Scientific Method With Kids
The scientific method is a process or method of research. A problem is identified, information about the problem is gathered, a hypothesis or question is formulated from the information, and the hypothesis is put to test with an experiment to prove or disprove its validity.
Sounds heavy… What in the world does that mean?!?
The scientific method can simply be used as a guide to help lead the discovery process. You don’t need to try and solve the world’s biggest science questions! The scientific method is all about studying and learning things right around you.
As kids develop practices that involve creating, gathering data, evaluating, analyzing, and communicating, they can apply these critical thinking skills to any situation.
💡To learn more about the scientific method and how to use it, CLICK HERE .
Even though the scientific method seems like it is just for big kids, this method can be used with kids of all ages! You can have a casual conversation with younger kids or do a more formal notebook entry with older kids!
Gummy Bear Science Fair Project
Science projects are an excellent way for older kids to show what they know about science. They can also be used in various environments, including classrooms, homeschools, and groups.
Kids can take everything they have learned about using the scientific method , stating a hypothesis, choosing variables , and analyzing and presenting data.
💡 Sample Hypothesis: If a gummy bear is placed in water overnight, then it will increase in size due to the process of osmosis, where water molecules move from an area of higher concentration (the water) to an area of lower concentration (inside the gummy bear).
Want to turn this gummy bear osmosis experiment into an awesome science fair project? Check out these helpful resources.
- Science Project Tips From A Teacher
- Science Fair Board Ideas
- Easy Science Fair Projects
More Fun Candy Science Experiments
- Try a candy taste test with chocolate.
- Why do the colors not mix in this skittles experiment?
- Dissolving candy corn experiment is fun to do !
- Make a coke and mentos eruption !
- What happens when you add pop rocks to soda?
- Try this floating M&M experiment.
Helpful Science Resources
Here are a few resources that will help you introduce science more effectively to your kiddos or students and feel confident yourself when presenting materials. You’ll find helpful free printables throughout.
- Best Science Practices (as it relates to the scientific method)
- Science Vocabulary
- 8 Science Books for Kids
- All About Scientists
- Science Supplies List
- Science Tools for Kids
Printable Science Projects For Kids
If you’re looking to grab all of our printable science projects in one convenient place plus exclusive worksheets and bonuses like a STEAM Project pack, our Science Project Pack is what you need! Over 300+ Pages!
- 90+ classic science activities with journal pages, supply lists, set up and process, and science information. NEW! Activity-specific observation pages!
- Best science practices posters and our original science method process folders for extra alternatives!
- Be a Collector activities pack introduces kids to the world of making collections through the eyes of a scientist. What will they collect first?
- Know the Words Science vocabulary pack includes flashcards, crosswords, and word searches that illuminate keywords in the experiments!
- My science journal writing prompts explore what it means to be a scientist!!
- Bonus STEAM Project Pack: Art meets science with doable projects!
- Bonus Quick Grab Packs for Biology, Earth Science, Chemistry, and Physics

Subscribe to receive a free 5-Day STEM Challenge Guide
~ projects to try now ~.


Gummy Bear Experiment
Osmosis can be a difficult concept for kids to understand. I’ve always found that visual explanations really hit home with kids and help them to understand. Today we have a growing gummy bear experiment that is a perfect compliment to our Gummy Mummy experiment that explores the science of desiccation and diffusion. Because gummy bears are made of gelatin they will not dissolve in water like other candy will. They will however absorb liquids and change in shape and size. We’ve set up an experiment with four different liquids to see the difference in how the gummy bears are able to absorb each and how they change over the course of the day.
Great Growing Gummies – Gummy Bear Osmosis Experiment
Table of Contents

Disclaimer: This article may contain commission or affiliate links. As an Amazon Influencer I earn from qualifying purchases. Not seeing our videos? Turn off any adblockers to ensure our video feed can be seen. Or visit our YouTube channel to see if the video has been uploaded there. We are slowly uploading our archives. Thanks!
What is Osmosis?
Scientifically, Osmosis is when solvent molecules (usually water) cross a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. This creates equilibrium between the solute and solvent, balancing the concentration of solutes on both sides of the membrane. Osmosis is a passive process in that it requires no energy from the cell to occur.
Now – that’s a lot of big words and concepts so let’s break down that vocabulary:
Solvent : substance able to dissolve other substances. Solute : a dissolved substance Membrane : a thin, soft flexible sheet or layer especially of a plant or animal part Semi-Permeable Membrane : a membrane that only allows certain substances to pass through. Concentration : the amount of a component in a given substance. Equilibrium : a state of adjustment between opposing or divergent influences or elements
Gummy Bear Osmosis Lab
Gummy Bears Small Clear Bowls or Jars Water Sparkling Water White Vinegar Oil
I like to start this in the morning so you can check on it throughout the day and see the changes in the gummy bears.
STEP 1: Lay out four bowls on the table and put a gummy bear in each bowl. Then beside each bowl put another gummy bear of the same color so you can compare the two easily over the course of the day.
STEP 2: Measure equal amounts of each of your solvents. We used a quarter of a cup of water, sparkling water, white vinegar and oil and poured them over the gummy bears in the bowl.

STEP 3: This is a great time to have a discussion about osmosis and have your kids make predictions about what they think is going to happen in each bowl and why. What effect might each substance have on the gummy bear? Have the kids write down their predictions.
STEP 4: Set a timer for an hour and let the bears do their thing.
STEP 5: Check back each hour for the rest of the day and write down observations over the course of the day.
Gummy Bear Osmosis Experiment Results
Now the exciting part… the results of our experiment! Let’s take a look at the results individually first.

When gummy bears are soaked in water the bear will swell and grow in size. This is because the water will flow into the gummy bear through its semi-permeable membrane. The sugar molecules try to spread and dissolve but they can’t get out of the gelatin so they expand resulting in the gummy bear expanding.
Sparkling Water

Will have a similar result to water. The only difference is that the addition of carbon dioxide to the water can have an acidic effect on the bears which would cause the outside to soften allowing more water to be able to pass through the bear and it swells up more. You will also be able to observe the carbon dioxide bubble sticking to the outside of the bear.
White Vinegar

White Vinegar will have an acidic reaction with the gummy bear softening the outside of it, however the liquid is not as easily absorbed into the bear as water so the gummy may get softer but will not change in size as much as the bears soaked in water.

Because oil is polar it doesn’t mix well with water or other substances. The oil will have very little effect on the bears and you will not see much change if any at all. This gummy bear will also retain its color the best because the oil isn’t breaking down the bear or being absorbed into it so the structure and color will remain the same.
Comparing the Results
The most fascinating part of this experiment is comparing the results of the different solvents. Set the gummy bears out side by side with their controls so you can visually see the differences.

To get really scientific with your results, which is perfect for your older kids or kids needing more of a challenge, have them weigh and measure the gummies and compare results with the controls and each other.
You can also dissect the gummy bears and view them under a microscope to look for microscopic changes.
Extension Ideas
I think your kids will love this Gummy Bear Lab experiment on Osmosis! Encourage your students to get creative and add other variations like adding things like salt or baking soda to the water to see if it changes the results. Or try other solvents.
Want more osmosis experiments? Try this Rainbow Water Beads Experiment or the Bouncy Egg Experiment which involves a chemical reaction and osmosis.
Want more gummy science? Check out our Gummy Mummies . Or make your own gummies! You can check out these recipes on the site: Valentine’s Day Gummies , Star Wars Gummies , Rainbow Dragon Egg Gummies .
5 Days of Smart STEM Ideas for Kids
Get started in STEM with easy, engaging activities.
Gummy Bear Science Experiments
Katelyn is a freelance writer and travel blogger who loves writing about travel, health, finance, and science topics. She holds a bachelor's and master's degree in science fields.
Learn about our Editorial Policy .
Mom always said never play with your food, but that wouldn't be any fun! Using fun food, like gummy bears, is a great tool to teach kids about the basics of chemistry.
The Amazing Growing Gummy Bear
The amazing growing gummy bear is a simple and fun experiment for children under the age of 12. The set up will take under an hour, but the experiment will run for at least 48 hours.
While most sugary candy dissolves in water, gummy bears are made with gelatin, which prevents the bears from dissolving. The gummy bear experiment is a great way to teach kids about osmosis. Osmosis is the process when water moves from a greater concentration of water to a lower concentration of water, such as the gummy bear. Try the experiment and see what happens!
- Gummy Bears
- Three glasses of water
- One tablespoon of salt
- One tablespoon of sugar
- Kitchen scale
- Paper towels
- Pen and paper
- Clock or timer
Instructions
- Select three gummy bears of the same color.
- Measure the length, height and width of each gummy bear and write it down.
- Weigh each gummy bear and write it down.
- Label each glass with its contents: water, salt water or sugar water.
- Fill the glass labeled water with one-half cup of plain water.
- Fill the glass with labeled salt water with one-half cup of water. Add and mix in one tablespoon of salt until all the salt has dissolved.
- Fill the glass labeled sugar water with one-half cup of water. Add and mix in one tablespoon of sugar until all the sugar has dissolved.
- Add a gummy bear to each glass and note the time.
- Wait 12 hours, measure and weigh each gummy bear.
- Replace the gummy bears back into their glasses.
- Check back after 24 hours, measure and weigh each gummy bear.
- Check back after 48 hours, measure and weigh each gummy bear.
How Does It Work?
What happened to the gummy bears? Why do they grow instead of dissolving like other candies? Gummy bears contain gelatin which is the same ingredient in Jell-O. Once the water and gelatin have cooled, the water in the gummy bears is drawn out leaving behind a delicious solid candy bear.
Gelatin is a long chain-like molecule that twists to create a solid form. When a gummy bear is placed in a glass of water, it becomes the solute. The solute is the dissolved material in the solution. The water is the solvent. Since the gummy bear does not contain water, when it is added to a glass of water, the water moves into the gummy bear by the process of osmosis.
Salt is a much smaller molecule than gelatin. There is more salt molecules in the water mixture than there are in the gummy. The water molecules will move towards the salt molecules to even out the number of water and salt molecules in the solution. That's why the gummy bear in the salt water doesn't grow that much if at all. What happened to the gummy bear in the sugar water?
The Amazing Growing Gummy Bear Part II
Now that the kids have learned what happens to the gummy bears in water and salt water, it's time to find out what gummy bears do in other solvents. The experiment doesn't need to be fancy, just find other liquids in the kitchen, like vinegar, milk, vegetable oil, or anything else that can be found in the pantry and refrigerator.
- Glasses or bowls
- Vegetable Oil
- Other liquids found in the kitchen (optional)
- Select three (or more depending on the number of solvents) gummy bears of the same color.
- Measure the length, height, and width of each gummy bear and write it down.
- Label each glass with its contents.
- Fill the glass labeled with its liquid contents.
- Add a gummy bear to each glass and start the timer.
Osmosis Made Easy
The amazing growing gummy bear experiment is a fun and simple experiment to teach children the basic principles of osmosis. By using colorful and delicious gummy bears, kids can see how water moves in and out of the bear. We just don't recommend eating the bears after they have been in salt water or vinegar!
STEM Little Explorers
Knowing through exploring.
Home » Articles » STEM » STEM Science » Gummy Bears Osmosis Experiment

Gummy Bears Osmosis Experiment
Today we will combine two fun activities from our childhood: eating gummy bears and learning about osmosis just kidding about osmosis being fun, back then it was a hard concept to grasp. but in today’s experiment, we will show you how to learn this important concept in a fun and easy way, article contents.
What is Osmosis?
Osmosis is defined as the movement of water molecules from a solution with a higher concentration of water molecules to a solution with a lower concentration of water molecules, through a cell’s semipermeable (partially permeable) membrane . What do we mean by the concentration of water? It’s the proportion of the water in a solution. Let’s talk about that next.
Solvent, Solute, and Solution
Speaking about Osmosis, you will probably often hear about solvent, solute, and solution. So let’s see what they are.
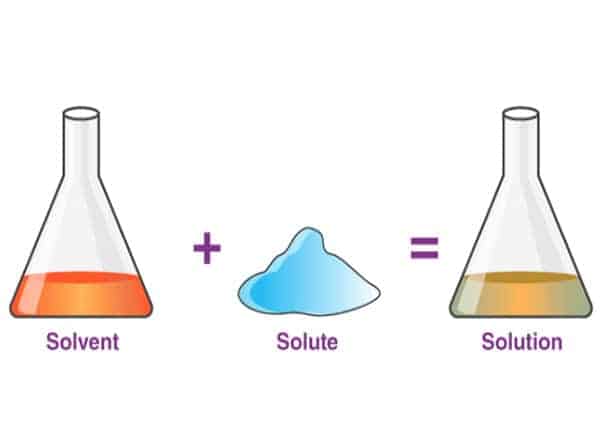
A solvent is any substance that dissolves other substances that we put in it. The most common solvent is water . We know that if we, for example, add sugar to the water, it will dissolve. This is important since, in our organism, water dissolves ions and proteins in our cells.
On the other hand, in our example above, the sugar would be a solute . The solute is a substance dissolved in another substance. So, sugar (solute) dissolves in water (solvent).
And the product we get is called a solution . Solutions can have different concentrations , depending on how much solute we dissolve in a solvent. If we add more sugar to the water, it will be sweeter and denser, more concentrated. However, this solution will now have a lower concentration of water molecules, since there are other things (sugar) in as well.
To summarise – when sugar (solute) dissolves in water (solvent) we get a mixture of water and sugar (solution) .
What is Semi-Permeable Membrane?
Think of the membrane as a wall with gaps (it’s semipermeable!). When solutions on both sides of the wall have the same concentration, nothing interesting happens – there is an equal probability water molecules will move from each side of the wall so in the end concentration will stay the same.
However, if we change the balance on one side of the wall, for example, add salt to one side – water molecules will now move from the place where there are more of them (ordinary water) to a place where there are fewer of them (salted water).
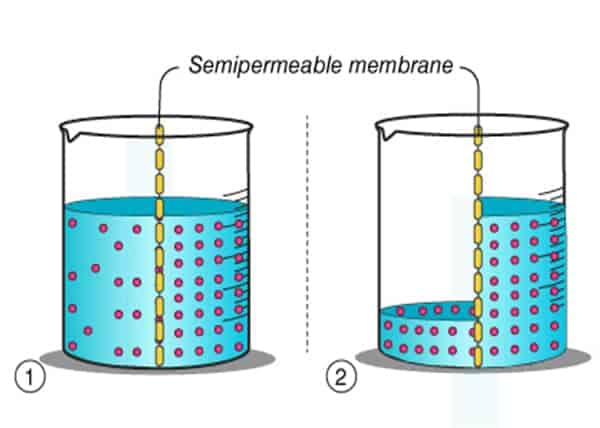
This state of different concentrations is also called osmotic pressure and therefore the amount of liquid will increase on the side with more salt, and decrease on the side where the salt concentration is lower until the osmotic pressure is equalized. The goal is to reach equilibrium, a state where concentrations are the same on both sides.
Here, we have 2 explanations of the process:
- The Mechanical explanation is that molecules of salt are blocking the movement of the water molecules so they are less likely to move from that side.
- The Chemical explanation is that salt molecules consist of ions – Na+ and Cl-. Since water molecules are also partially charged they are attracted to salt molecules and therefore don’t move through the membrane.
Why Is Osmosis Important?
Osmosis is essential for the survival of all living organisms . It allows nutrients and minerals to move inside the cells, through the cell membrane, and also for waste to move out of the cells. For example, plants absorb water from the earth through the process of osmosis.
Try to remember the last time you ate something salty, such as chips. You must have been very thirsty afterward. This is because salt prevents water from passing into the cell through the semipermeable membrane and no matter how much you drink, it is difficult to quench your thirst.
Let’s go now and demonstrate the osmosis process in a simple way using gummy bear candies and different solutions.
Materials needed for the Gummy Bear Experiment

- Gummy bears (gummy candies) . You can buy gummy candy in any grocery shop. We have used Haribo gummy bears and they worked well for our experiment. It is not important which gummy candy you use, but we have got reports that some types/brands of gummy candy won’t work well and will just dissolve. Best to have at least 4 gummy bears to make easy comparisons of all experimental results and the original gummy bear.
- Water . 2 deciliters of water will be enough. We will add 1 deciliter to 2 of our glasses.
- Salt . One tablespoon of salt will be enough to act as a solvent and create a concentrated solution.
- Vinegar . We will need 1 deciliter of vinegar to serve us as the second solution and we will add it to the last glass.
- 3 glasses . Since we will have 3 experimental groups, we will need 3 glasses. In the first glass, we will add pure water. In the second glass, we will add water and salt. And in the third glass, we will add vinegar.
Instructions for making Gummy Bear Osmosis Experiment
Check the video at the beginning of the article to see how to conduct this experiment. As mentioned in the required materials section, we used three types of solvent (water, salted water, and vinegar) but you can experiment with any type of solvent.
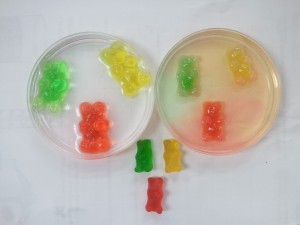
- Prepare 4 gummy bears (one for every type of solvent, +1 for comparison). Gummy bears are excellent for this experiment because they are made out of sugar, water, and gelatine. Gelatine doesn’t dissolve in water, but it allows water to pass through so it functions as a semipermeable membrane.
- Prepare your solvents. Put pure water in one glass, water with a big spoon of salt into the second glass, and vinegar into the third glass. 1 deciliter of liquid in each glass will be more than enough. You can also experiment with different mixtures, like oil, milk, or soda to see what will happen.
- Put 1 gummy bear into each solution . Leave one gummy bear on the side so you can compare afterward. Leave the gummy bears inside their solutions for a few hours. Check every 3 hours to see the changes.
Results of the osmosis experiment

- After 9 hours, we observed that the gummy bear left in pure water got much bigger than in the other solutions. The water went in! There is just a little bit of water in the gummy bear, so there was big osmotic pressure.
- Gummy bear in salted water got just a little bit bigger . Osmosis at work! Salted water had a lower concentration of water than the pure one, so in this situation, less water went into the gummy bear.
- In vinegar, the gummy bear got bigger , but it also started to fall apart, and that’s because of the acid in vinegar which can dissolve the gelatine.
What kind of solutions did you use and what are the results? Tell us all about your experiment in the comments!
What will you develop and learn?
- Knowledge from chemistry and biology . Osmosis, semipermeable membrane, solutions, etc., all play a big role in the functioning of living organisms. Talking about them will help us in better understanding what is happening on the cell level.
- What is osmosis and how does it work. Without osmosis, there would not be life. So understanding osmosis is important to understand biology.
- Scientific method and conducting experiments. Here, we conducted a scientific experiment with 3 experimental variables (water, salted water, vinegar) and a control variable (gummy bear that we didn’t put into any solution). This enabled us to control every aspect that could influence the outcome of the experiment.
- Learning by doing . We best learn through experience, and here, we conduct our own experiments. So new knowledge while having fun is guaranteed!
We hope you too were enjoying this experiment. If you are in the mood for more great activities, we have some to recommend.
- If you are interested in learning about defusion, a similar process to osmosis, then you can check How to demonstrate diffusion with hot and cold water article.
- We also recommend learning about oxidation and how oxygen reacts with electrons in the Apple oxidation experiment .
- If you are interested in making your own sweet candy, you can learn How to make homemade sugar crystals (Rock Candy) .
- And finally, if you are interested in learning about polarity, the chemical property of atoms, you can learn about it in a simple but fun Colorful milk polarity experiment .
Happy experimenting!
If you’re searching for some great STEM Activities for Kids and Child development tips, you’re in the right place! Check the Categories below to find the right activity for you.

STEM Science
Videos, guides and explanations about STEM Science in a step-by-step way with materials you probably already have at your home. Find new Science ideas.

STEM Technology
Videos, guides and explanations about STEM Technology in a step-by-step way with materials you probably already have at your home. Find new Technology ideas.

STEM Engineering
Videos, guides and explanations about STEM Engineering in a step-by-step way with materials you probably already have at your home. New Engineering ideas!

Videos, guides and explanations about STEM Math in a step-by-step way with materials you probably already have at your home. Find new Mathematics ideas.

Find out all about development psychology topics that you always wanted to know. Here are articles from child psychology and development psychology overall.

First year of Child’s Life
Following a Child’s development every month from its birth. Personal experiences and tips on how to cope with challenges that you will face in parenting.
5 thoughts on “ Gummy Bears Osmosis Experiment ”
- Pingback: 5 Amazing Balloon Experiments - STEM Little Explorers
- Pingback: How to Shrink a Bag with Microwaves? - STEM Little Explorers
- Pingback: How to Make Apple Oxidation Experiment | STEM Little Explorers
- Pingback: 100 Awesome Chemistry Experiments For All Ages -
- Pingback: Demonstrate Density with Orange Density Experiment | STEM Little Explorers
Leave a Reply Cancel reply
You must be logged in to post a comment.
Get Fresh news from STEM fields
I'm not interested in STEM

IMAGES
COMMENTS
Jan 2, 2023 · Instructions for Gummy Bear Experiment First, label each glass for a different kind of liquid (tap water, salt water, sugar water, milk, vinegar, etc.) Then, add a half cup of water to the tap water glass.
Nov 21, 2015 · The gummy bear experiment is a fun activity that teaches the basic concept of osmosis to the little ones in an easy manner. They will also be thrilled at the idea that their favorite gummy bears could teach them a lesson or two in science. Gummy Bear Experiment Gummy Bear Science Project Instructions Hypothesis for Growing/Shrinking Gummy Bears
It is time for these little bears to grow up...and out with this gummy bear science project! Watch as gummy bears grow and shrink in different liquids in this kid-friendly experiment. This project is open for exploration and discovery, so kick things off by asking your child what they will happen to a gummy bear in water.
Gummy Bear Experiment Sheet (included at the end of this post, although the spacing is slightly different) Gummy Bear Scientific Data Table (included at the bottom of this post) Instructions for the Gummy Bear Osmosis Experiment. Label each glass with its contents: water, salt water, sugar water, etc. Fill the glass labeled water with one-half ...
May 4, 2020 · Instructions. 1. Fill two of your bowls with cool water. ... Plop one gummy bear into each bowl. ... If you have time (and gummies) to spare, you can elaborate on this experiment by testing ...
Carefully take out the gummy bears from their respective containers and place them on a plate, or the Gummy Bear Experiment Worksheet if you are using it. Place a control gummy bear (one that has not been submerged in any liquid) next to each of the gummy bears you removed from the containers. Discuss with your child the difference in sizes.
Nov 10, 2024 · Set Up A Gummy Bear Osmosis Lab. Let’s find out what liquid makes gummy bears grow the biggest! Remember, the dependent variable is the size of the gummy bears and the independent variable is the liquid you use. Learn more about variables in science. Supplies: Gummy bears ; 4 cups; water ; baking soda; vinegar; ruler or measuring scale; salt ...
Oct 6, 2024 · Today we have a growing gummy bear experiment that is a perfect compliment to our Gummy Mummy experiment that explores the science of desiccation and diffusion. Because gummy bears are made of gelatin they will not dissolve in water like other candy will. They will however absorb liquids and change in shape and size.
The Amazing Growing Gummy Bear . The amazing growing gummy bear is a simple and fun experiment for children under the age of 12. The set up will take under an hour, but the experiment will run for at least 48 hours. While most sugary candy dissolves in water, gummy bears are made with gelatin, which prevents the bears from dissolving.
Jan 12, 2022 · Instructions for making Gummy Bear Osmosis Experiment Check the video at the beginning of the article to see how to conduct this experiment. As mentioned in the required materials section, we used three types of solvent (water, salted water, and vinegar) but you can experiment with any type of solvent.