An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Research Programs & Contacts

Clinical Research in Type 2 Diabetes
Studies in humans aimed at the prevention, treatment, and diagnosis of Type 2 Diabetes and the mechanistic aspects of its etiology.
The Clinical Research in Type 2 Diabetes (T2D) program supports human studies across the lifespan aimed at understanding, preventing and treating T2D. This program includes clinical trials that test pharmacologic, behavioral, surgical or practice-level approaches to the treatment and/or prevention of T2D, including promoting the preservation of beta cell function. Studies may also advance the development of new surrogate markers for use in clinical trials. Studies can be designed to understand the pathophysiology of T2D, including the role of gestational diabetes and metabolic imprinting on the development of T2D, as well as factors influencing the response to treatment. The program also encompasses epidemiologic studies that improve our understanding of the natural history and pathogenesis of T2D, and the development of diagnostic criteria to distinguish type 1 and type 2 diabetes, especially in the pediatric population. The program also supports research to understand and test approaches to accelerate the translation of efficacious interventions into real-world practice and adoption; and to address health equity by reducing health disparities in the incidence and/or clinical outcomes of T2D.
NIDDK Program Staff
- Shavon Artis Dickerson, Dr.P.H., M.P.H. Health Equity and Implementation Science; Centers for Diabetes Translation Research (P30) Program
- Henry B. Burch, M.D. Clinical studies utilizing existing digital health technology for the prevention and treatment of type 2 diabetes, clinical and basic science studies involving non-neoplastic disorders of the thyroid, clinical studies involving medical and novel dietary treatment of type 2 diabetes.
- Maureen Monaghan Center, Ph.D., CDCES Health Psychology, Behavioral Science, Clinical Management of Diabetes
- Minnjuan Flournoy Floyd, Ph.D., M.P.H., M.B.A. Health Disparities and Health Equity; specifically adult T2D Health Equity Research; clinical research in T2D
- Jean M. Lawrence, Sc.D., M.P.H., MSW Type 2 diabetes risk and prevention after gestational diabetes; Studies of adults with diabetes/pre-diabetes using secondary data and observational designs, and natural experiments
- Hanyu Liang, M.D., Ph.D. Hepatic Metabolism; Insulin Resistance; Type 2 Diabetes; Obesity; Bariatric Surgery
- Barbara Linder, M.D., Ph.D. Type 2 diabetes in children and youth; human studies of metabolic imprinting
- Saul Malozowski, M.D., Ph.D., M.B.A. Neuroendocrinology of hypothalamic-pituitary axis, neuropeptide signaling and receptors; hormonal regulation of bone and mineral metabolism; HIV/AIDS-associated metabolic and endocrine dysfunction
- Pamela L. Thornton, Ph.D.
- Theresa Teslovich Woo, Ph.D. Human behavior, developmental cognitive neuroscience, and brain-based mechanisms involved in obesity and diabetes
Recent Funding Opportunities
Dissemination and implementation research in health (r01 clinical trial optional), precision medicine for type 1 diabetic nephropathy (u01 clinical trial not allowed), heal initiative: studies to enable analgesic discovery (r61/r33 - clinical trial not allowed), cellular models of hiv pathogenesis within niddk mission areas (r01 clinical trial not allowed), research resource for human organs and tissues (u42 clinical trial not allowed), related links.
View related clinical trials from ClinicalTrials.gov.
Study sections conduct initial peer review of applications in a designated scientific area. Visit the NIH’s Center for Scientific Review website to search for study sections.
Research Resources
NIDDK makes publicly supported resources, data sets, and studies available to researchers to accelerate the rate and lower the cost of new discoveries.
- Ancillary Studies to Major Ongoing Clinical Studies to extend our knowledge of the diseases being studied by the parent study investigators under a defined protocol or to study diseases and conditions not within the original scope of the parent study but within the mission of the NIDDK.
- NIDDK Central Repository for access to clinical resources including data and biospecimens from NIDDK-funded studies.
- NIDDK Information Network (dkNET) for simultaneous search of digital resources, including multiple datasets and biomedical resources relevant to the mission of the NIDDK.
Additional Research Programs
Research training.
NIDDK supports the training and career development of medical and graduate students, postdoctoral fellows, and physician scientists through institutional and individual grants.
Diversity Programs
The NIDDK offers and participates in a variety of opportunities for trainees and researchers from communities underrepresented in the biomedical research enterprise. These opportunities include travel and scholarship awards, research supplements, small clinical grants, high school and undergraduate programs, and a network of minority health research investigators.
Small Business
Small business programs.
NIDDK participates in the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs. These programs support innovative research conducted by small businesses that has the potential for commercialization.
Human Subjects Research
NIDDK provides funding for pivotal clinical research, from preliminary clinical feasibility to large multi-center studies.
Translational Research
NIDDK provides funding opportunities and resources to encourage translation of basic discoveries into novel therapeutics.
Meetings & Workshops

Jan. 13 - 14, 2025 Bethesda, MD

April 3 - 4, 2025 Bethesda, MD

Supports researchers with tools to enhance scientific rigor, reproducibility, and transparency, and provides a big data knowledge base for genomic and pathway hypothesis generation.

Learn about current projects and view funding opportunities sponsored by the NIH Common Fund .
Registration is required at eRA Commons and grants.gov and can take 4 weeks.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Type 2 diabetes articles from across Nature Portfolio
Type 2 diabetes mellitus, the most frequent subtype of diabetes, is a disease characterized by high levels of blood glucose (hyperglycaemia). It arises from a resistance to and relative deficiency of the pancreatic β-cell hormone insulin.
Latest Research and Reviews
Genetic basis of early onset and progression of type 2 diabetes in South Asians
In a cohort of 50,556 South Asian individuals, partitioned polygenic scores helped identify genetic susceptibility to insulin deficiency and unfavorable fat distribution as key drivers of young-onset T2D diagnosis and faster progression to diabetes-related complications.
- Sam Hodgson
- Alice Williamson
- Sarah Finer
Revisited guidelines for metabolic tolerance tests in mice
In this Review, the authors examine current guidelines for metabolic tolerance tests in mice and provide a set of revisited recommendations to improve the reproducibility and clinical translation of the findings.
- Cedric Moro
- Christophe Magnan
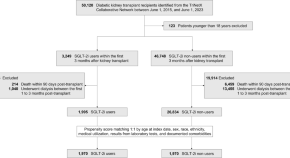
The outcomes of SGLT-2 inhibitor utilization in diabetic kidney transplant recipients
Sodium-glucose cotransporter 2 inhibitors (SGLT-2i) have demonstrated efficacy in reducing cardiovascular events and potentially improving kidney function in diabetic patients. Here, using the TriNetX platform, the authors show that SGLT-2 inhibitors reduce all-cause mortality and major adverse cardiac and kidney events in diabetic kidney transplant recipients.
- Jia-Yuh Sheu
- Li-Yang Chang
- Vin-Cent Wu
Healthy food diversity and the risk of major chronic diseases in the EPIC-Potsdam study
- Daniela V. Nickel
- Franziska Jannasch
- Matthias B. Schulze
Analysis of metabolites associated with ADIPOQ genotypes in individuals with type 2 diabetes mellitus
- Tainá Gomes Diniz
- Caroline Severo de Assis
- Darlene Camati Persuhn
Intake of animal and plant proteins and risk of all-cause mortality in patients with type 2 diabetes: results from NHANES
- Ahmad Jayedi
- Mahdieh-Sadat Zargar
News and Comment
T h 1 responses in type 2 diabetes.
- Stephanie Houston
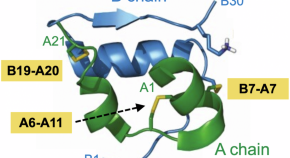
Splitting the chains: ultra-basal insulin analog uncovers a redox mechanism of hormone clearance
Reporting in Nature Communications , Kjeldsen and colleagues describe a redox mechanism of insulin clearance based on separation of A- and B chains. Exploiting an ultra-long-acting analog protected from classical clearance pathways, the study highlights principles of protein stability in pharmacology.
- Michael A. Weiss
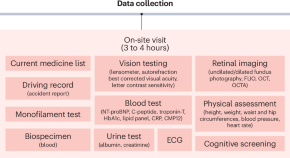
AI-READI: rethinking AI data collection, preparation and sharing in diabetes research and beyond
Here, we introduce Artificial Intelligence Ready and Equitable Atlas for Diabetes Insights (AI-READI), a multidisciplinary data-generation project designed to create and share a multimodal dataset optimized for artificial intelligence research in type 2 diabetes mellitus.
- Sally L. Baxter
- Virginia R. de Sa
- Xujing Wang
Genetics brings new insight to β-cell function
A meta-analysis of genome-wide association study for eight traits related to pancreatic β-cell function, based on 26,000 individuals, identified 55 independent association signals mapping to 44 loci. This study highlighted new effectors of β-cell function.
- Amélie Bonnefond
- Philippe Froguel
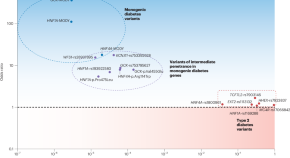
Parsing the spectrum of allelic architectures in diabetes
Distinguishing ordinary diabetes from its monogenic forms has been one of the challenges in optimally managing the disease. Using high-quality imputation of rare variants and large databases, a study now defines the gray zone between the two and lays down a blueprint for objectively evaluating the related variants.
- Constantin Polychronakos
Blood lipid profiling indicates that dietary fat quality is associated with cardiometabolic risk
Dietary guidelines advise substituting saturated fats with unsaturated fats. We used detailed blood fat composition profiling in diet trials and population studies to confirm that a moderate high-fat diet from plant oils is better for metabolism and heart health than a diet with similar fat levels from animal sources.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Glycemia reduction in type 2 diabetes—Hypoglycemia outcomes: A randomized clinical trial
Contributed equally to this work with: Elizabeth R. Seaquist, Lawrence S. Phillips
Roles Data curation, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Medicine, Division of Diabetes and Endocrinology, University of Minnesota Medical School, Minneapolis, MN, United States of America
Roles Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing
Affiliation Department of Medicine, Atlanta VA Medical Center, Decatur, GA and Division of Endocrinology and Metabolism, Emory University School of Medicine, Atlanta, GA, United States of America
Roles Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Writing – original draft, Writing – review & editing
Affiliation Department of Biostatistics and Bioinformatics, Milken Institute of Public Health, The Biostatistics Center, The George Washington University, Rockville, MD, United States of America
Roles Investigation, Project administration, Supervision, Writing – review & editing
Affiliation Department of Medicine, Division of Endocrinology, Metabolism and Diabetes, University of Colorado School of Medicine, Aurora, CO, United States of America
Roles Conceptualization, Data curation, Investigation, Writing – review & editing
Affiliation Health Partners Institute, International Diabetes Center, Minneapolis, MN, United States of America
Roles Data curation, Investigation, Project administration, Supervision, Writing – review & editing
Affiliation Division of Endocrinology and Fleischer Institute for Diabetes & Metabolism, Albert Einstein College of Medicine, Bronx, NY, United States of America
Affiliation Departments of Medicine and Pediatrics, Naomi Berrie Diabetes Center, Columbia University, New York, NY, United States of America
Roles Conceptualization, Data curation, Investigation, Project administration, Supervision, Writing – review & editing
Roles Data curation, Investigation, Supervision, Writing – review & editing
Affiliation Department of Veterans Affairs Pacific Islands Health Care System, Honolulu, HI, United States of America
Roles Data curation, Investigation, Writing – review & editing
Affiliation Clinical Trials Unit, Pennington Biomedical Research Center, Baton Rouge, LA, United States of America
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing
Affiliation University of Texas—Southwestern Medical Center, Dallas, TX, United States of America
Roles Data curation, Writing – review & editing
Affiliations Geriatric Research Education and Clinical Center, Bruce W. Carter Veterans Affairs Center, Miami, FL, United States of America, Department of Public Health Sciences, University of Miami, Miami, FL, United States of America, Robert Stempel College of Public Health, Florida International University, University Park, FL, United States of America, Endocrinology & Metabolism Institute, Center for Geriatric Medicine, Cleveland Clinic, OH, United States of America
Affiliation University of Michigan, Ann Arbor, MI, United States of America
Roles Data curation, Formal analysis, Validation, Visualization, Writing – review & editing
- [ ... ],
¶ Membership of the GRADE Research Group is provided in S1 Text
- [ view all ]
- [ view less ]
- Elizabeth R. Seaquist,
- Lawrence S. Phillips,
- Alokananda Ghosh,
- Chelsea Baker,
- Richard M. Bergenstal,
- Jill P. Crandall,
- Robin S. Goland,
- Michaela R. Gramzinski,
- Sophia H. Hox,

- Published: November 15, 2024
- https://doi.org/10.1371/journal.pone.0309907
- Reader Comments
Hypoglycemia is a major concern in type 2 diabetes (T2DM), but little is known about its likelihood compared across common therapies. We compared the likelihood of hypoglycemia among metformin-treated patients with T2DM randomized to the addition of one of 4 common therapies.
Research design & methods
Randomized, controlled trial of 5,047 participants with T2DM of <10 years’ duration, hemoglobin A1c (HbA1c) 6.8–8.5% (50.8–69.4 mmol/mol). Randomization to addition of glargine U100, glimepiride, liraglutide, or sitagliptin over 5.0 ± 1.3 (mean ± SD) years. HbA1c was measured quarterly; if a level >7.5% (>58.5 mmol/mol) was confirmed, rescue glargine and/or aspart insulin was added. We conducted a per-protocol analysis of 4,830, who attended at least one post-baseline visit and took at least one dose of assigned study medication. We assessed severe hypoglycemia events reported throughout the entire study. At quarterly visits, all participants were asked about hypoglycemic symptoms within the last 30 days, and those in the glargine and glimepiride groups were asked for any measured glucose <70 mg/dL (3.9 mmol/L) within this time period.
While participants were taking their assigned medications, severe hypoglycemia occurred in 10 (0.8%), 16 (1.3%), 6 (0.5%), and 4 (0.3%), (p<0.05) and hypoglycemic symptoms in 659 (54.2%), 833 (68.3%), 375 (32.4%), and 361 (29.1%) of participants following randomization to glargine, glimepiride, liraglutide, and sitagliptin, respectively (p<0.001).
Conclusions
In metformin-treated patients with T2DM who add a second medication, hypoglycemia is most likely with addition of glimepiride, less with glargine, and least likely with liraglutide and sitagliptin.
Trial registration
ClinicalTrials.gov Identifier: NCT01794143 .
Citation: Seaquist ER, Phillips LS, Ghosh A, Baker C, Bergenstal RM, Crandall JP, et al. (2024) Glycemia reduction in type 2 diabetes—Hypoglycemia outcomes: A randomized clinical trial. PLoS ONE 19(11): e0309907. https://doi.org/10.1371/journal.pone.0309907
Editor: Yasunori Sato, Keio University School of Medicine, JAPAN
Received: February 26, 2024; Accepted: July 24, 2024; Published: November 15, 2024
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Data Availability: The Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This manuscript is based on follow-up data and outcome assessments from the 5047 participants enrolled into the study. This database will be available in the NIDDK Central Repository by 2024. https://repository.niddk.nih.gov/home/ .
Funding: The GRADE Study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under Award Number U01DK098246. https://www.niddk.nih.gov/ The planning of GRADE was supported by a U34 planning grant from the NIDDK (U34-DK-088043). https://www.niddk.nih.gov/ The American Diabetes Association supported the initial planning meeting for the U34 proposal. https://diabetes.org/ The National Heart, Lung, and Blood Institute https://www.nhlbi.nih.gov/and the Centers for Disease Control and Prevention also provided funding support. https://www.cdc.gov/ The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The GRADE Study Research Group is deeply grateful to our participants whose loyal dedication made GRADE possible. It is true that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: Outside the submitted work, Dr. Elizabeth Seaquist reports grants or contracts from JDRF to her institution; consulting fees from Lily, NovoNordisk, Sanofi, and Zucara; honoraria from International Hypoglycemia study group; board membership with the American Diabetes Association; and receipt of sensors from Dexcom to her institution. Dr. Lawrence Phillips reports salary support from the Veterans Health Administration during the conduct of the study; grants from Janssen Pharmaceuticals, grants from Merck, Amylin, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, AbbVie, Vascular Pharmaceuticals, GlaxoSmithKline, Pfizer, AstraZeneca, Kowa, and Cystic Fibrosis Foundation; other support from DIASYST, outside the submitted work. Outside the submitted work, Dr. Richard M. Bergenstal reports consulting fees from Abbot Diabetes Care, Ascencia, Bigfoot Biomedical, Inc., DexCom, MannKind, Medtronic, Novo Nordisk, Sanofi, and United Health Care made to HealthPartners Institute; payments or honoraria from Sanofi and Vertex Pharmaceuticals made to Health Partners Institute; support for meetings or travel from Abbott Diabetes Care, Ascensia, CeQur, Eli Lilly, Embecta, MannKind, Novo Nordisk, Roche GmbH, Sanofi, Vertex Pharmaceuticals, and Zealand Pharma made to Health Partners Institute, and participation on a data safety monitoring or advisory board from Abbott Diabetes Care, CeQur, Eli Lilly, Embecta, Hygieia, Roche GmbH, and Zealand Pharma with payments made to HealthPartners Institute. Outside the submitted work, Dr. Jill Crandall reports non-financial support from Abbott. Outside the submitted work, Mary L Johnson reports research grants paid to Health Partners Institute from Sanofi, Novo Nordisk, and Lilly. Dr. Alokananda Ghosh, Dr. Naji Younes, Dr. John Lachin, Chelsea Baker, Dr. Robin S. Goland, Michaela R Gramzinski, Dr. Daniel S Hsia, Dr. Sophia H Hox, Dr. Philip Raskin, Dr. Willy M Valencia, and Andrea H Waltje have nothing to disclose. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Introduction
Hypoglycemia can be the limiting factor in achieving acceptable target glycemic control in patients with diabetes. Hypoglycemia can be serious–more common than hyperglycemia as a cause of hospital admissions among Medicare beneficiaries in 1999–2011 [ 1 ], and hypoglycemia related to use of insulin or sulfonylureas was among the most frequent causes of drug-related adverse events in emergency departments in 2013–2014 [ 2 ]. Although less common, hypoglycemia has also been reported with standard dosages of metformin, liraglutide and sitagliptin monotherapy [ 3 – 5 ]. The relative risk of experiencing hypoglycemia with different classes of drugs used to treat type 2 diabetes has not been extensively studied. In the CAROLINA trial, subjects randomized to glipizide had significantly more hypoglycemia than did those randomized to linagliptin and in UKPDS those randomized to sulfonylurea [glipizide or chlorpropamide) plus ultralente insulin had less hypoglycemia than did those randomized to ultralente insulin alone [ 6 , 7 ]. Our understanding of the likelihood of hypoglycemia with commonly used drugs would be enhanced by high-quality, prospective studies.
The Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) permits such a direct comparison. In GRADE, hypoglycemia was assessed in participants with type 2 diabetes treated only with metformin and then randomized to the addition of insulin glargine U100, glimepiride, liraglutide, or sitagliptin [ 8 , 9 ]. Participants were queried about episodes of hypoglycemia and related symptoms at regular intervals. As previously reported, severe hypoglycemia was more common in those randomized to glargine or glimepiride compared to liraglutide or sitagliptin [ 8 ]. In this analysis, we expand our observations to examine the comparative likelihood of different categories of hypoglycemic events across treatment groups in a randomized, controlled trial where medications were used as in usual clinical practice.
Research design and methods
As described previously [ 8 , 9 ], GRADE examined the addition of a long-acting insulin (glargine U100), a long-acting sulfonylurea (glimepiride), a glucagon-like peptide-1 receptor agonist (GLP-1 RA, liraglutide), or a dipeptidyl peptidase-4 inhibitor (DPP4, sitagliptin), in people with type 2 diabetes who were taking only metformin at baseline. Eligibility included duration of diabetes <10 years, age >30 years at diagnosis (>20 years in Native Americans), and baseline hemoglobin A1c (HbA1c) 6.8–8.5% (50.8–69.4 mmol/mol). Additional details on randomization and masking are provided in S2 Text . Institutional Review Board approval was obtained at each participating institution, and all participants gave written informed consent. Recruitment began May 1, 2013, and ended August 31, 2017.
Study medications were randomly assigned, and glargine and glimepiride were titrated with protocol-defined algorithms based on self-monitored blood glucose (SMBG) levels, aimed to achieve pre-breakfast glucose levels of 80–130 mg/dL (4.4–7.2 mmol/L) without hypoglycemic symptoms, or up to the maximum approved dose, whichever dose was lower. Liraglutide was titrated to 1.8 mg/day, unless limited by tolerability, and the sitagliptin dose was based on renal function according to the package insert. HbA1c was measured quarterly and if a value of > 7.5% (>58.5 mmol/mol) was confirmed, those randomized to glimepiride, liraglutide, or sitagliptin added “rescue” glargine, while those randomized to glargine added pre-prandial insulin aspart. Participants in the glargine and glimepiride groups were expected to perform SMBG with study-supplied strips and meters. During periods of glargine dose titration, participants were asked to check blood glucose in the morning when fasting. During periods of glimepiride dose titration, participants were asked to check blood glucose one to two times per day. Once doses of glargine and glimepiride were stabilized, both groups were asked to check blood glucose at least twice a week. Participants in these groups were also asked to perform SMBG if they had symptoms of hypoglycemia. At the discretion of study staff, SMBG could be performed more frequently and also used in the liraglutide and sitagliptin groups. SMBG was also implemented in participants who were given insulin after failing to maintain HbA1c ≤ 7.5% (≤ 58.5 mmol/mol).
We enrolled 5,047 participants and report findings from a per-protocol subset of 4,830 (95.7%) participants, which excludes 217 participants who never took their assigned medication or never attended follow-up visits. The median observation period was 3.8 (range 0–7.4) years, 51% of the participants had at least 4 years and 68% had at least 3 years of follow-up (see CONSORT diagram, Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0309907.g001
Hypoglycemia definition
The primary hypoglycemia outcome in GRADE was adjudicated severe hypoglycemia. All reports of severe hypoglycemia were collected throughout the study. In addition, at quarterly visits, all participants were asked about hypoglycemic symptoms within the last 30 days, and those in the glargine and glimepiride groups were asked for any measured glucose ≤70 mg/dL (3.9 mmol/L) within this time period. All reports of severe hypoglycemia triggered an adjudication process by investigators masked to treatment assignment ( S3 Text ). Only those episodes that were adjudicated as severe are included in this report.
Statistical analysis
We conducted a "per-protocol" analysis of episodes of hypoglycemia occurring while participants were taking only metformin and their assigned study medication. This analysis was limited to the subset of participants who attended at least one post-baseline visit and took at least one dose of their assigned study medication (N = 4,830), and we censored any episode of hypoglycemia that occurred after the addition of rescue insulin, after any use of non-study diabetes medication, or the discontinuation of the assigned treatment. For categorical variables, differences in the counts and percentages by treatment group were evaluated with a chi-squared p-value comparing the groups. For continuous variables, differences in the mean ± standard deviation were evaluated with an F-test p-value comparing the groups.
The probability that a participant reported hypoglycemia at a quarterly visit was estimated using a logistic Generalized Estimating Equations (GEE) model with treatment group as the sole covariate, and a first-order autoregressive (AR(1)) correlation structure. The estimated probabilities within each assigned medication treatment group (expressed as a percentage) are reported along with their 95% asymptotic confidence interval. Kaplan-Meier (KM) cumulative incidence plots with log-rank tests were used to compare the four treatment arms for time to first severe hypoglycemia.
To assess the likelihood of hypoglycemia within each of seven pre-specified subgroups (age, sex, race, ethnicity, HbA1c, BMI, and diabetes duration), we again calculated the probability of a participant reporting hypoglycemic symptoms at a quarterly visit using logistic GEE models, with each medication treatment group, a single subgroup variable and a subgroup by treatment interaction. The estimated probability (expressed as a percentage) within treatment group and subgroup strata and its 95% asymptotic confidence interval are reported. The p-value is that of the treatment by subgroup interaction term in the GEE model using a multivariate Wald test.
All analyses were conducted with R 4.2.1 (R Core Team 2022). All tests were two-sided, with statistical significance set at p<0.05. All p-values were nominal with no adjustment for multiple comparisons.
Institutional Review Board approval was obtained at each participating institution, and all participants gave written informed consent. GRADE is a multi-center RCT, approved by each clinical site’s institutional review boards. Please refer to S1 Table for information on each clinical site’s review board. We have indicated the primary review board submission information from the most recent annual renewal: The George Washington University. Office of Human Research—Institutional Review Board; IRB Number: 071245; Last Approved: 7/25/2023; Expires: 8/23/2024.
The 4,830 per-protocol participants randomized to the glargine, glimepiride, liraglutide and sitagliptin treatment groups had similar characteristics at baseline [ 8 , 9 ]. The cohort was 36.1% female, 66.3% White, 19.4% Black, and 18.3% Hispanic with an average age of 57.1 years, body mass index (BMI) 34.3 kg/m 2 , duration of diabetes 4.2 years, and HbA1c 7.5% (58.4 mmol/mol) ( Table 1 ).
https://doi.org/10.1371/journal.pone.0309907.t001
While participants were taking their assigned study medications, severe hypoglycemia occurred in 10 (0.8%), 16 (1.3%), 6 (0.5%), and 4 (0.3%) participants randomized to glargine, glimepiride, liraglutide, and sitagliptin, respectively (p<0.05). Hypoglycemic symptoms occurred in 659 (54.2%), 833 (68.3%), 375 (32.4%), and 361 (29.1%), p<0.001, of the glargine, glimepiride, liraglutide, and sitagliptin groups, respectively. In the glargine and glimepiride groups, any hypoglycemia (severe hypoglycemia, hypoglycemia symptoms or measured glucose < 70 mg/dL) occurred in 765 (62.9%) and 915 (75.1%), respectively, p<0.001. Among the four treatment groups, the probability of hypoglycemic symptoms being reported at a quarterly visit was higher with glimepiride than the other groups (18.8% vs. 10.6% with glargine, 5.1% with liraglutide, and 5.0% with sitagliptin [ Table 2 ]). A similar pattern was seen for severe hypoglycemia, albeit with much lower probabilities.
https://doi.org/10.1371/journal.pone.0309907.t002
Since participants could experience multiple episodes of hypoglycemia of varying severity and type during the study, the rows in Table 2 are not mutually exclusive.
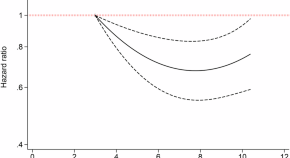
Fig 2 shows the cumulative incidence of severe hypoglycemia. There was both more severe hypoglycemia and hypoglycemia occurring earlier in the study in those assigned to glimepiride compared to glargine, and less in those assigned to liraglutide and sitagliptin.
The numbers plotted below the x-axis are the participants at risk for severe hypoglycemia at each follow-up time point by adjudication (i.e., the number of participants who were not adjudicated as having had severe hypoglycemia by that time).
https://doi.org/10.1371/journal.pone.0309907.g002
Of note, hypoglycemic symptoms were reported by participants in all groups ( Table 2 ). Participants in the glargine and glimepiride groups measured their blood glucose in 77% of the episodes in which they had symptoms, and most of these symptomatic episodes were associated with blood glucose values less than 70 mg/dL. Close to one third of the participants in the liraglutide and sitagliptin groups (32.4% and 29.1%, respectively) reported hypoglycemic symptoms, but many did not have corresponding glucose values since they were not provided with testing supplies.
Subgroup analyses ( Table 3 ) were conducted to assess potential impact on treatment group differences in the likelihood of reporting hypoglycemic symptoms at a quarterly visit, stratified by seven baseline factors. Probabilities (expressed as percentages) within treatment groups and among strata are presented. The pattern of treatment group differences varied across HbA1c tertiles’ strata (p = 0.04 for test of interaction). Among those in the lowest HbA1c tertile (6.8–7.2%), the likelihood was lower with glargine or glimepiride (8.7% for glargine, 18.6% for glimepiride) than for the other treatment groups (around 5.0%). None of the other factors (age, sex, etc.) showed heterogeneity among strata.
https://doi.org/10.1371/journal.pone.0309907.t003
In this study of 4,830 participants with type 2 diabetes taking only metformin at baseline and randomized to addition of glargine, glimepiride, liraglutide, or sitagliptin, we found that while participants were on their assigned medication, there was significantly more severe hypoglycemia in those assigned to glimepiride than to glargine. Both medications had higher rates than with liraglutide or sitagliptin.
Severe hypoglycemia was rare, but hypoglycemic symptoms were common in the GRADE cohort. More than half of the participants assigned to addition of glargine and glimepiride and >25% of the participants in the liraglutide and sitagliptin groups reported these symptoms while they were taking only metformin and their assigned study medication. Since the symptoms participants report are not specific for hypoglycemia, a report of symptoms could lead to classification error if not confirmed with a glucose measurement. Only participants in the glargine and glimepiride groups were given glucose testing materials and provided with instructions for when to use them prior to meeting the HbA1c >7.5% (>58.5 mmol/mol) outcome. Thus, we cannot determine if the symptoms reported by participants randomized to liraglutide and sitagliptin truly reflect low glucose levels. More accurate ascertainment of hypoglycemia in future studies would ideally include more extensive confirmatory glucose testing.
Seven pre-specified baseline factors were examined to determine their potential contributions to the likelihood of reporting hypoglycemia symptoms at a quarterly visit, and only HbA1c was found to have a statistically significant interaction. This differs from results in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study, where there was an increased risk of severe hypoglycemia in women and African Americans, and with advanced age, and higher baseline HbA1c [ 10 ]. However, ACCORD has not reported an association between baseline factors and the likelihood of hypoglycemic symptoms, and in GRADE the number of severe hypoglycemic events was too small to find a statistical relationship with baseline characteristics. Perhaps the reason we saw less hypoglycemia in those with the lowest baseline HbA1c levels is that they needed low dose glimepiride or glargine to achieve the treatment target.
Use of sulfonylureas and insulin carries a greater risk of hypoglycemia than diabetes medications acting via alternative mechanisms, including GLP-1 RAs such as liraglutide and DPP4 inhibitors such as sitagliptin [ 11 , 12 ]. However, most previous comparisons have not classified hypoglycemia across a spectrum from severe hypoglycemia to symptoms alone. Our finding that severe hypoglycemia and hypoglycemic symptoms were less common with liraglutide and sitagliptin than with glargine and glimepiride is consistent with previous reports [ 11 , 12 ] and clinical experience. This observation, when coupled with the finding that those randomized to liraglutide experienced less weight gain than those randomized to other medications and had an efficacy essentially equal to glargine and better than sitagliptin and glimepiride [ 8 ], provides further evidence that liraglutide might be considered as a first line agent to add on top of metformin in adults with type 2 diabetes of less than 10 years in duration. The presence of severe hypoglycemia in participants treated with liraglutide or sitagliptin was unexpected and provides evidence that this outcome can occur when these drugs are used in combination with metformin. However, there may have been other causes for hypoglycemia that were not captured by our adjudication process.
The strengths of this study include its large sample size; nationwide participation; and inclusion of individuals who may be at increased risk for iatrogenic hypoglycemia, including age 60+ years, lower level of education, and members of underrepresented racial/ethnic groups [ 10 ]. Further, treatment regimens were consistent with current clinical practice, and hypoglycemia was ascertained, categorized, and adjudicated using standardized procedures. The large sample size and successful randomization permitted a robust secondary analysis of an important clinical question.
Limitations include reliance on participant self-report for ascertainment of hypoglycemia, and potential undercounting of episodes since non-severe hypoglycemic events were only collected for the 30 days prior to the quarterly visits. However, recall bias was likely reduced by restricting the assessment to the 30-day window. Also, timing and cost precluded study of sodium-glucose cotransporter-2 (SGLT-2) inhibitors [ 9 ]. Therefore, GRADE cannot provide insight into the impact of this agent compared to the four study medications.
In conclusion, in adults with type 2 diabetes of less than 10 years duration using metformin monotherapy, the addition of glimepiride was associated with a greater risk of severe hypoglycemia by adjudication than addition of glargine, and both were associated with a greater risk than addition of liraglutide or sitagliptin. Parallel results were observed with less severe categories of hypoglycemia. If limiting the risk of hypoglycemia is a priority when optimizing glycemic management in patients with type 2 diabetes who are using only metformin, clinicians should consider adding a GLP-1 RA such as liraglutide, which has a low likelihood of hypoglycemia and high efficacy of glucose lowering [ 8 ]. If a sulfonylurea or insulin is needed, there may be less hypoglycemia with addition of glargine, compared to addition of glimepiride.
Supporting information
S1 checklist..
https://doi.org/10.1371/journal.pone.0309907.s001
S1 Text. GRADE research group listing.
https://doi.org/10.1371/journal.pone.0309907.s002
S2 Text. GRADE masking and random assignment.
https://doi.org/10.1371/journal.pone.0309907.s003
S3 Text. Hypoglycemia data collection and definitions.
A. Hypoglycemia data collection. B. Severe hypoglycemia adjudication. C. Hypoglycemia definitions.
https://doi.org/10.1371/journal.pone.0309907.s004
S1 Table. IRB approval information.
https://doi.org/10.1371/journal.pone.0309907.s005
Acknowledgments
GRADE: The Department of Veterans Affairs provided resources and facilities. Additional support was provided by grant numbers P30 DK017047, P30 DK020541-44, P30 DK020572, P30 DK072476, P30 DK079626, P30 DK092926, U54 GM104940, UL1 TR000439, UL1 TR000445, UL1 TR001108, UL1 TR001409, 2UL1TR001425, UL1 TR001449, UL1 TR002243, UL1 TR002345, UL1 TR002378, UL1 TR002489, UL1 TR002529, UL1 TR002535, UL1 TR002537, 2UL1 TR001425 and UL1 TR002548. Educational materials were provided by the National Diabetes Education Program. Material support in the form of donated medications and supplies were provided by Becton, Dickinson and Company, Bristol-Myers Squibb, Merck & Co., Inc., Novo Nordisk, Roche Diagnostics, and Sanofi. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The GRADE Study Research Group is deeply grateful to our participants whose loyal dedication made GRADE possible.
Guarantors Statement: Naji Younes and Alokananda Ghosh are the guarantors of this work and as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: Outside the submitted work, Dr. Elizabeth Seaquist reports grants or contracts from JDRF to her institution; consulting fees from Lily, NovoNordisk, Sanofi, and Zucara; honoraria from International Hypoglycemia study group; board membership with the American Diabetes Association; and receipt of sensors from Dexcom to her institution. Dr. Lawrence Phillips reports salary support from the Veterans Health Administration during the conduct of the study; grants from Janssen Pharmaceuticals, grants from Merck, Amylin, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, AbbVie, Vascular Pharmaceuticals, GlaxoSmithKline, Pfizer, AstraZeneca, Kowa, and Cystic Fibrosis Foundation; other support from DIASYST, outside the submitted work. Outside the submitted work, Dr. Richard M. Bergenstal reports consulting fees from Abbot Diabetes Care, Ascencia, Bigfoot Biomedical, Inc., DexCom, MannKind, Medtronic, Novo Nordisk, Sanofi, and United Health Care made to HealthPartners Institute; payments or honoraria from Sanofi and Vertex Pharmaceuticals made to Health Partners Institute; support for meetings or travel from Abbott Diabetes Care, Ascensia, CeQur, Eli Lilly, Embecta, MannKind, Novo Nordisk, Roche GmbH, Sanofi, Vertex Pharmaceuticals, and Zealand Pharma made to Health Partners Institute, and participation on a data safety monitoring or advisory board from Abbott Diabetes Care, CeQur, Eli Lilly, Embecta, Hygieia, Roche GmbH, and Zealand Pharma with payments made to HealthPartners Institute. Outside the submitted work, Dr. Jill Crandall reports non-financial support from Abbott. Outside the submitted work, Mary L Johnson reports research grants paid to Health Partners Institute from Sanofi, Novo Nordisk, and Lilly. Dr. Alokananda Ghosh, Dr. Naji Younes, Dr. John Lachin, Chelsea Baker, Dr. Robin S. Goland, Michaela R Gramzinski, Dr. Daniel S Hsia, Dr. Sophia H Hox, Dr. Philip Raskin, Dr. Willy M Valencia, and Andrea H Waltje have nothing to disclose.
Prior Presentation: The abstract for this manuscript was presented at the American Diabetes Association’s 82nd Scientific Sessions in New Orleans, Louisiana, June 3–7, 2022, and at the International Hypoglycemia Study Group 2 nd Annual Advances in Hypoglycemia Conference, Virtual Meeting, November 16–17, 2022.
- View Article
- PubMed/NCBI
- Google Scholar
Diabetes Type 2 clinical trials at UCSD
10 in progress, 4 open to eligible people
Developing and Testing a Self-Compassion Tool Kit to Improve the Care of Individuals With Type 2 Diabetes
open to eligible people ages 18 years and up
There is a high prevalence of anxiety and depression in patients with Type 2 Diabetes (T2D). While past studies demonstrate the potential therapeutic effect of mindfulness-based interventions in patients with T2D, little is understood about the mode of delivery or quantity of the intervention necessary to experience benefits. This project aims to develop and implement a self-compassion tool kit based on the principles of mindfulness and meditation to better understand how self-compassion works to affect psychological health and wellbeing in patients with T2D. The investigators will study the impact of a self-compassion tool kit - including mindfulness meditation, exercise, journaling and sleep parameters - on T2D. Enhancing emotional well-being could complement current T2D treatments to facilitate improved quality of life.
San Diego , California
More Fresh Fruit and Vegetable Prescription Program for Families with Type 2 Diabetes Mellitus
open to eligible people ages up to 18 years
Rady Children's Hospital San Diego (RCHSD), UCSD Division of Child and Community Health and the Center for Community Health, and Northgate Gonzalez (NG) Markets will collaborate to create a Produce Prescription Program (Fruit and Vegetable Prescription Program) to be implemented in the RCHSD Diabetes Clinic. We will provide families on Medi-Cal who have a child with T2DM with a fruit and vegetable prescription (FVRx) which will enhance their ability to purchase GusNIP-eligible fresh fruits and vegetables (FV). These prescriptions will be delivered in the form of an electronic voucher that can be filled at any NG Markets throughout San Diego and Riverside counties. The goal of this program is to increase the purchase and consumption of fresh fruits and vegetables, decrease food insecurity, and improve metabolic outcomes for children with type 2 diabetes mellitus (T2DM).
Semaglutide Treatment in the Real-world for Fibrosis Due to NAFLD in Obesity and T2DM
open to eligible people ages 40-79
Conduct a community intervention study that will 1) validate a screening approach to identify patients at risk for advanced NAFLD in the obese or T2DM population, and 2) test whether semaglutide treatment is effective for the management of significant fibrosis due to NAFLD in high-risk patients.
La Jolla , California
Time-Restricted Eating for Type II Diabetes: TRE-T2D
open to eligible people ages 18-75
This is a randomized clinical trial to assess the feasibility and efficacy of time-restricted eating (TRE) to improve glucose regulation and cardiovascular health of participants with type 2 diabetes mellitus (T2DM). Participants will be randomized into 2 groups: 1) standard of care (SOC), in which they will continue to follow their physician's treatment plan, or 2) SOC and TRE (8-10 hours eating window).
Tirzepatide (LY3298176) Compared With Dulaglutide on Major Cardiovascular Events in Participants With Type 2 Diabetes
Sorry, in progress, not accepting new patients
The purpose of the trial is to assess the efficacy and safety of tirzepatide to dulaglutide in participants with type 2 diabetes and increased cardiovascular risk.
La Jolla , California and other locations
Afrezza® INHALE-1 Study in Pediatrics
INHALE-1 is a Phase 3, open-label, randomized clinical study evaluating the efficacy and safety of Afrezza in combination with a basal insulin (i.e., the Afrezza group) versus insulin aspart, insulin lispro or insulin glulisine in combination with a basal insulin (i.e., the Rapid-acting Insulin Analog [RAA] injection group) in pediatric subjects with type 1 or type 2 diabetes mellitus. Following 26 weeks of randomized treatment (i.e., Afrezza or RAA injection combined with a basal insulin), all subjects will enter a treatment extension where subjects will receive Afrezza until Week 52. The purpose of the treatment extension is to assess safety and efficacy with continued use of Afrezza. Pediatric subjects ≥4 and <18 years of age will be enrolled in this study. Subjects will be randomly assigned in a 1:1 ratio to either the Afrezza group or the RAA injection group. The study is composed of: - Up to 5-week screening/run-in period - 26 week randomized treatment period - 26-week treatment extension - 4-week follow-up period
San Diego , California and other locations
CGM for Management of Type 2 Diabetes in Pregnancy
Sorry, not yet accepting patients
The goal of this clinical trial is to learn if continuous glucose monitoring works better than self-monitoring of blood glucose (fingersticks) to treat type 2 diabetes in pregnancy. It will also learn about all risk factors (biologic, personal, social) for maternal and infant complications in type 2 diabetes pregnancies. The main questions it aims to answer are: 1. Does continuous glucose monitoring improve infant outcomes compared to self-monitoring of blood glucose? 2. Does continuous glucose monitoring improve maternal diabetes control and other maternal outcomes compared to self-monitoring of blood glucose? 3. What other factors increase the risk of maternal and infant complications? Participants will: 1. Use continuous glucose monitoring or self-monitoring of blood glucose to monitor blood sugar control from enrollment until delivery 2. Have blood drawn at enrollment, 24 weeks, 34 weeks and delivery to measure hemoglobin A1c levels and store blood for future analysis 3. Complete surveys about social support, environmental stressors, diabetes distress and glucose monitoring satisfaction at research visits 4. Have umbilical cord blood collected at delivery for analysis
THC Effects on Glucose in Type 2 Diabetes
This study will examine the effects THC has on Glucose Metabolism and Endothelial Functioning in participants with Type 2 Diabetes. The participants will complete blood tests and tests to measure energy expenditure, CVD risks, and glucose metabolism. These tests will be performed prior to start of treatment and again after 2-weeks of treatment with the THC or placebo.
AI Ready and Equitable Atlas for Diabetes Insights
Sorry, accepting new patients by invitation only
The study will collect a cross-sectional dataset of 4000 people across the US from diverse racial/ethnic groups who are either 1) healthy, or 2) belong in one of the three stages of diabetes severity (pre-diabetes/diet controlled, oral medication and/or non-insulin-injectable medication controlled, or insulin dependent), forming a total of four groups of patients. Clinical data (social determinants of health surveys, continuous glucose monitoring data, biomarkers, genetic data, retinal imaging, cognitive testing, etc.) will be collected. The purpose of this project is data generation to allow future creation of artificial intelligence/machine learning (AI/ML) algorithms aimed at defining disease trajectories and underlying genetic links in different racial/ethnic cohorts. A smaller subgroup of participants will be invited to come for a follow-up visit in year 4 of the project (longitudinal arm of the study). Data will be placed in an open-source repository and samples will be sent to the study sample repository and used for future research.
Diabetes Prevention Program Outcomes Study AD/ADRD Project
The DPPOS AD/ADRD project will address the overarching question: What are the determinants and the nature of cognitive impairment among persons with pre-diabetes (PreD) and type 2 diabetes (T2D), who are a high-risk group for cognitive impairment and represent a large fraction of the United States (US) population? This U19 proposal addresses the National Alzheimer's Project Act goal to "prevent, halt, or reverse AD" in the high-risk group of persons with pre-diabetes and type 2 diabetes, who represent over half of the population aged 60 years and older in the US.
Our lead scientists for Diabetes Type 2 research studies include Jeremy Pettus, MD Pam Taub, MD Edward C Chao, DO Kyung Rhee, MD, MS, MA .
Last updated: November 14, 2024

COMMENTS
A Study of the Metabolome and Microbiome Following Bariatric Surgery, and the Effect on Metabolism in Patients with Type 2 Diabetes Rochester, MN . The purpose of this study is to identify changes to the metabolome (range of chemicals produced in the body) and microbiome (intestine microbe environment) that are unique to Roux-en-Y gastric bypass surgery and assess the associated effect on the ...
The new research, published in the journal Nature Communications, offers a potential strategy for developing new therapies that could restore dysfunctional pancreatic beta-cells or, perhaps, even prevent Type 2 diabetes from developing. The new study shows that the beta-cells of Type 2 diabetes patients are deficient in a cell trafficking ...
The Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study is following more than 5,000 people across the country who have type 2 diabetes to find out which combination of two diabetes medicines is best for blood glucose, also called blood sugar, management; has the fewest side effects; and is the most helpful for overall ...
The purpose of the Glycemia Reduction Approaches in Type 2 Diabetes: A Comparative Effectiveness (GRADE) Study was to examine the relative effectiveness of agents from four of the most commonly ...
NIDDK Program Staff. Shavon Artis Dickerson, Dr.P.H., M.P.H. Health Equity and Implementation Science; Centers for Diabetes Translation Research (P30) Program Henry B. Burch, M.D. Clinical studies utilizing existing digital health technology for the prevention and treatment of type 2 diabetes, clinical and basic science studies involving non-neoplastic disorders of the thyroid, clinical ...
Type 2 diabetes mellitus, the most frequent subtype of diabetes, is a disease characterized by high levels of blood glucose (hyperglycaemia). It arises from a resistance to and relative deficiency ...
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1) receptor agonist that is under development for the treatment of type 2 diabetes. The efficacy ...
The primary aim of the randomized Glycemia Reduction Approaches in Type 2 Diabetes: A Comparative Effectiveness (GRADE) Study was to compare the effectiveness of agents from four commonly used ...
Objective Hypoglycemia is a major concern in type 2 diabetes (T2DM), but little is known about its likelihood compared across common therapies. We compared the likelihood of hypoglycemia among metformin-treated patients with T2DM randomized to the addition of one of 4 common therapies. Research design & methods Randomized, controlled trial of 5,047 participants with T2DM of <10 years ...
The study will collect a cross-sectional dataset of 4000 people across the US from diverse racial/ethnic groups who are either 1) healthy, or 2) belong in one of the three stages of diabetes severity (pre-diabetes/diet controlled, oral medication and/or non-insulin-injectable medication controlled, or insulin dependent), forming a total of four groups of patients.