- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information

A-case-study-on-acute-gastritis-with-moderate-dehydration-nursing-care-plan-and-discharge-teachings compress
Maternal child nursing (ncm 107), lorma colleges.
Recommended for you
Students also viewed.
- Transcultural Nursing Across the Lifespan Transcultural Perspective in Childbearing
- Ncp template
- Group 4 Algorithm Optimal feeding of the clinically stable baby weighing less than 2500 g
- Nursing Care of a Family Experiencing a Postpartal Complication
- IMCI 2 5 assessment form Worksheet 1
- IMCI 0 2 months assessment form Worksheet
Related documents
- NCP 1 - NCP
- Care-plan-on-pneumonia compress
- Acute-gastritis compress
- CASE- Study-CVA- Vertigo
- Copy of DIKW- Exercise - i need to have a based in this
- Major EXAM OB WARD - Maternal and Child exam
Preview text
St. anthony college of roxas city, san roque extension, roxas city, college of nursing, - acute gastritis -, (a case study), in partial fulfillment, of the requirements in, rle ncm 116, submitted by:, sarah l. rodaje s, submitted to:, nita a. ammogao, r., may 13, 2021, san roque extension, roxas city, capiz 5800, college of nursing, ii. table of content, title page..............................................................................................................., table of content..................................................................................................., introduction.........................................................................................................., objectives............................................................................................................., textbook discussion............................................................................................., anatomy and physiology......................................................................................, pathophysiology..................................................................................................., vital information..................................................................................................., laboratory and diagnostic test............................................................................, drug tabulation....................................................................................................., nursing care plan.................................................................................................., discharge planning................................................................................................, acknowledgement................................................................................................., bibliography..........................................................................................................., iv. objectives, general objectives, this case study seeks to demonstrate the student’s knowledge regarding the, general health and disease condition of a patient with diagnosis, its disease, process, possible complications, treatment plan and nursing interventions., specific objectives, demonstrate adept observation skills by being able to identify signs and, symptoms manifested in acute gastritis with moderate dehydration., accurately present a thorough general assessment of the client which includes, clinical and physical assessment taking., provide patient health teachings to the family of the client., acquire adequate knowledge on acute gastritis with moderate dehydration., understand the pathophysiology of the case being presented., understand the role of drug therapy in managing the client’s diagnosis., be able to recognize the client’s condition and advocate appropriate nursing, interactions., encourage interaction to the family of the patient to establish rapport., apply the vincentian core values in planning the nursing care., v. textbook discussion, gastritis is a term which encompasses a series of conditions that present with, inflammation of the gastric mucosa. it is classified based on a time course as either, acute or chronic., acute gastritis is the inflammation of the gastric mucosa lasting several hours, to a few days while chronic gastritis is a prolonged inflammation of the stomach, that may be caused by either benign of malignant ulcers of the stomach and bacteria., dietary indiscretion – most often cause of acute gastritis., other causes of acute gastritis – include overuse of aspirin and other non-, steroidal anti-inflammatory drugs (nsaids), excessive alcohol intake, bile reflux, and radiation therapy. a more severe form of acute gastritis is caused by the, ingestion of strong acid or alkali, which may cause the mucosa to become, gangrenous or to perforate., helicobacter pylori infection – it evolved to penetrate the mucoid lining of, the stomach in order to penetrate the gastric mucus lining of the stomach and, thereby establish infection., sign and symptoms, some patient may not show signs of gastritis but when they do, they may exhibit:, vomiting – food contents or blood, indigestion, vi. anatomy and physiology, the stomach is a muscular organ located on the left side of the upper abdomen. the, stomach receives food from the esophagus. as food reaches the end of esophagus, it, enters the stomach through a muscular valve called the lower esophageal sphincter., the stomach secretes acid and enzymes that digest food. the stomach muscles, contract periodically, churning food to enhance digestion., 4 regions of the stomach, cardia – is the first part of the stomach below the esophagus. it contains the, cardiac sphincter, which is a thin ring of muscle that helps to prevent stomach, contents from going back up into the esophagus., fundus- it is located inferior in the diaphragm. it is the rounded are that lies, to the left of the cardia., body- is the largest and, main part of the stomach., this is where food is mixed, and starts to break down., pylorus- is the part of the, stomach that connects to, the small intestine. this, region includes the pyloric sphincter, which is a thick ring of muscle that acts as, valve to control the emptying of stomach contents into the duodenum. the, pyloric sphincter also prevents the contents of the duodenum from going back, into the stomach., layers of the stomach wall, mucosa - has glands that produce stomach acid and other important, compounds. one example is the enzyme pepsin. while the stomach acid breaks, down food and protects it from infection, pepsin breaks down protein., submucosa- it is the next layer that covers the mucosa. it is made up of, connective tissue that contains larger blood and lymph vessels, nerve cells and, fibers. the blood supply of the submucosa provides nutrients to the wall of the, muscularis propria - is the next layer that covers the submucosa. it is the, main muscle of the stomach and its function is to move and mix the stomach, serosa - is the fibrous membrane that covers the outside of the stomach. it, has a smooth, slippery surface and secrets a thin, watery secretion known as, serous fluid. the smooth, wet surface of the serosa helps to protect the, stomach from friction as it expands with food and mixes to mix and propel the, viii. vital information, name of the patient: a. m., age: 5 years and 6 months old, sex: female, citizenship: filipino, religion: roman catholic, date of birth: september 8, 2016, chief complaint: vomiting, date admitted: february 8, 2021, admitting diagnosis: acute gastritis with moderate dhn, attending physician: dr. c., final diagnosis: acute gastritis with moderate dhn, source of information: mother and chart, clinical assessment, past medical history, the patient was admitted last october 2019 at roxas memorial provincial hospital due, to complaints of cough and fever and the admitting/final diagnosis of pediatric, community acquired pneumonia type c (pcap c). the patient has a complete history, of immunizations., present medical history, hours prior to admission, the client experienced abdominal pain and vomiting of prior, ingested food and two more episodes. the mother brought the child to saint anthony, college of roxas city inc (hospital) on february 8, 2021, 9:05 pm for admission and, was admitted due to the chief complaint of abdominal pain and vomiting and the, admitting diagnosis of acute gastritis with moderate dehydration with the following, hrs pta – 3 am – (+) abdominal pain, hrs pta – 7 pm – (+) vomiting of prior ingested food and 2 more episodes, family history, the grandmother at the matriarchal side of the client has hypertension while the, grandfather is diabetic. on the patriarchal side of the client, both the grandmother and, grandfather have hypertension which carried over to some of their children, specifically, the first and third child. the rest of the family members are well and alive., physical assessment, parts/system, finds interpretation, skin inspection skin appears warm to touch, and presence of rashes., hair inspection hair is black in color. no, presence of lice., head inspection head is normocephalic and, symmetrical., no presence of lesions., nails inspection, nails are trimmed and pale in, color. smooth and intact with, hearing: both ears can hear sounds with good auditory acuity to normal voice., mental status: oriented to people with short span of attention., emotional status: has separation anxiety from her mother and being irritable., patient female male, well and alive, 2 years old, ix. laboratory and diagnostic test, macroscopic, color pale straw, transparency slightly hazy, sugar negative, bilirubin negative, ketone negative, specific gravity 1., blood negative, protein negative, urobilinogen normal, nitrite negative, leuko esterase negative, microscopic, amphorous phosphates, wbc 0-2 / hpf, epithelial cells 0-2 / hpf, bacteria few, result reference values significance, hematocrit 0 vol (fr) 0 – 0 normal, becoming dehydrated., encouraged patient to wash her, diet any foods that could aggravate the, symptoms should be eliminated. foods such, as milk, tea, colas and chocolate should be, consumed in small amounts or eliminated if, possible. bedtime eating should be avoided, as it increases nocturnal acid secretions., spirituality encouraged patient to thank god, despite all of the circumstances, they’ve experienced., told client to pray every night before, outpatient follow-up follow-up care on february 15, 2021, (11:00 am) at sach opd, xiii. acknowledgement, no one who achieves success does so without acknowledging the help of, others. the wise and confident acknowledge this help with gratitude., -alfred north whitehead-, the completion of this case study could not have been possible without the, participation and assistance of some whose names may not all be enumerated. their, help is sincerely full of gratitude from yours truly. however, i would like to express my, deepest gratitude to the following., to the lord almighty, the giver of life and wisdom, source of physical and spiritual, strength, knowledge and ability and opportunity to make this case study and to, persevere and complete it. without his blessings, this success would have not been, to my dear parents who unconditionally and wholeheartedly support my dreams and, goals in life, their willingness to invest and participate on my academic performances,, without them i may not be able to continue and pursue this profession., to ms. rubilyn sumaylo, rn, msn., dean of the college of nursing, for making this, rotation possible, thus allowing us to further enhance and strengthen our knowledge,, skills, and attitude and for lifting our spirits during the backbreaking rotations., to mrs. nita a. ammogao, rn., our clinical instructor for this last rotation, for her, endless support, kindness and understanding during the rotation, for sharing her, knowledge and expertise, experiences and words of encouragement., to mrs. ethel bergantinos, rn, msn., mrs. maureen tajolosa rn., mrs. edrelyn, venturanza rn., msn., and mrs. betty s. miranda, rn., man., our clinical instructors, from the previous rotations, for imparting their skills and experiences, kindness and, patience and for honing the student nurses’ skills as well., to my classmates who are always understanding and responsive whenever i have, some clarifications and questions, for their contribution and effort to complete this, case study, remained patient and determined in times of difficulty and endured the, pressure, for encouraging one another, and for the sacrifices made for the completion, of this case study., acute gastritis is the inflammation of the gastric mucosa lasting several hours to a few, chronic gastritis, chronic gastritis is a prolonged inflammation of the stomach that may be caused by, either benign of malignant ulcers of the stomach and bacteria., helicobacter pylori (h. pylori), helicobacter pylori (h. pylori) bacteria are a common cause of digestive illnesses,, including gastritis (the irritation and inflammation of the stomach lining), peptic ulcers, (sores in the lining of the stomach, small intestine, or esophagus), and even stomach, cancer later in life., non-steroidal anti-inflammatory drugs (nsaids), non-steroidal anti-inflammatory drugs (nsaids) are medicines that are widely used to, relieve pain, reduce inflammation, and bring down a high temperature. they're often, used to relieve symptoms of headaches, painful periods, sprains and strains, colds and, flu, arthritis, and other causes of long-term pain..
- Multiple Choice
Course : Maternal Child Nursing (NCM 107)
University : lorma colleges.

- Discover more from: Maternal Child Nursing NCM 107 Lorma Colleges 43 Documents Go to course
- More from: Maternal Child Nursing NCM 107 Lorma Colleges 43 Documents Go to course
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Emphysematous gastritis: A case series of three patients managed conservatively
Hassan nasser, tommy ivanics, shravan leonard-murali, dania shakaroun, ann woodward.
- Author information
- Article notes
- Copyright and License information
Corresponding author. [email protected] [email protected]
Received 2019 Jul 11; Accepted 2019 Sep 30; Collection date 2019.
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Computed tomography scan is the best test to establish the diagnosis of EG.
Early recognition and initiation of therapy is crucial to prevent progression of EG.
Surgical exploration is indicated after failure of non-operative management.
Keywords: Case series, Emphysematous gastritis, Gastric emphysema, Gastritis, Computed tomography
Introduction
Emphysematous gastritis (EG) is a rare condition characterized by air within the gastric wall with signs of systemic toxicity. The optimal management for this condition and the role of surgery is still unclear. We here report three cases of EG successfully managed non-operatively.
Presentation of cases
All three of our patients were elderly females with several co- morbidities. The chief presenting symptom was abdominal pain with signs of systemic toxicity ranging from tachycardia and hypotension to acute kidney injury. Computed tomography (CT) scan revealed gastric pneumatosis in all patients. One patient had extensive portal venous gas, and another had free intraperitoneal air. All patients were managed with nothing by mouth, proton pump inhibitors, intravenous fluid resuscitation, and antibiotics. Repeat CT scan in two patients in 3–4 days demonstrated resolution of the pneumatosis. They were all discharged home tolerating an oral diet.
The presentation of EG is non-specific and the diagnosis is primarily established by findings of intramural air in the stomach on CT scan. The initial management of EG should be nothing by mouth, proton pump inhibitor, intravenous fluid resuscitation, and antibiotics with surgical exploration only reserved for cases that fail non-operative management, demonstrate clinical deterioration, or develop signs of peritonitis.
Early recognition and initiation of appropriate therapy is crucial to prevent the progression of EG. EG, even in the presence of portal venous air or pneumoperitoneum, should not represent a sole indication for surgical exploration and trial of initial non-operative management should be attempted when clinically appropriate.
1. Introduction
Emphysematous gastritis (EG) is a rare infection of the stomach wall by invasive gas-forming organisms. Gram-positive, gram-negative, anaerobic, and fungal organisms have been implicated in the pathogenesis of EG and commonly isolated organisms include Streptococcus species, Escherichia coli , Enterobacter species, Clostridium species, Staphylococcus aureus , Klebsiella pneumoniae , Pseudomonas aeruginosa , and Candida species [ 1 , 2 ]. In 42.4% of cases however, no organism is identified [ 3 ]. In a recently published review, Watson et al. reported 59 cases of EG up to June 2014 in the English literature with a reported mortality rate of 47.5% [ 3 ]. Predisposing factors for EG include malignancy, caustic ingestion, recent surgery, bowel obstruction, gastric distension, emesis, steroids, immunosuppressive medications, chemotherapy, alcohol, and nonsteroidal ant-inflammatory drugs [ [1] , [2] , [3] , [4] ]. EG typically presents with abdominal pain, nausea, vomiting, diarrhea, and occasionally hematemesis and sepsis [ 5 , 6 ]. Computed tomography (CT) scan is the most effective diagnostic modality to detect intramural emphysema [ 7 ]. Several studies emphasize the distinction between EG and gastric emphysema (GE). GE is reported to be a relatively benign condition where gas is seen in the wall of the stomach and is thought to occur secondary to barotrauma with no associated signs of infection or systemic toxicity [ 4 ]. Nevertheless, because of the overlap in the presentation between EG and GE, distinguishing between these conditions may be challenging [ 1 , 5 ] Over the last two decades, the role of surgical exploration in patients with EG has been questioned and the optimal treatment strategy remains unclear [ 3 ]. We report three cases of EG which were managed non-operatively without any mortality. This case series has been reported in line with the PROCESS guidelines [ 8 ].
2. Presentation of cases
This is a retrospective case series which involves three patients who presented to our academic institution between March 2018 and June 2018 with emphysematous gastritis. This study was exempt from ethical approval at our institution.
2.1. Case 1
A 78-year-old female presented to an outside hospital with one-week history of diffuse abdominal pain with associated nausea, vomiting, and diarrhea. Her past medical history was significant for factor V Leiden, deep vein thrombosis, pulmonary embolism, hypertension, diabetes mellitus, morbid obesity, atrial fibrillation, heart failure with preserved ejection fraction, and chronic obstructive pulmonary disease on 2 liters home oxygen. She was on warfarin and had an inferior vena cava filter in place for her history of venous thromboembolic disease and thrombophilia. Her vital signs were significant for tachycardia. Abdominal examination was soft, non-distended, minimally tender to palpation in the epigastrium without rebound or guarding. Initial laboratory studies were significant for a white blood cell count (WBC) of 21,600 cells/μL, serum creatinine of 2.18 mg/dL, lactate of 2.7 mmol/L, and international normalized ratio (INR) of 5.36. Her INR was corrected with vitamin K and fresh frozen plasma and the patient was transitioned to an intravenous unfractionated heparin infusion. CT scan of the abdomen without contrast was obtained to evaluate her abdominal pain and revealed gastric distension with gastric pneumatosis ( Fig. 1 ). She was treated for EG with nasogastric tube (NGT) decompression, intravenous fluid resuscitation, nil per os (NPO), antibiotics (vancomycin, cefepime, metronidazole, and fluconazole), and proton pump inhibitor (PPI). Two days after admission, she developed bloody output through her NGT and dropped her hemoglobin from 11.2 to 7.8 g/dL. Her heparin infusion was stopped. At this point, she was transferred to our medical intensive care unit for escalation of care. On arrival the patient was hemodynamically stable with coffee-ground output from her NGT. She reported that her abdominal pain had improved significantly. Laboratory studies eventually revealed a normalization of her WBC count, serum creatinine, and lactic acid. Her hemoglobin stabilized without further need for transfusion. Decision was made to not pursue an esophagogastroduodenoscopy (EGD) given no further evidence of bleeding and improvement in her pain. General surgery was consulted and recommended non-operative management. After four days from the first CT scan, a CT angiography of the abdomen with contrast was obtained to evaluate for vascular patency which revealed patent celiac trunk and resolution of her gastric pneumatosis ( Fig. 2 ). The NGT was then removed and the patient tolerated an oral diet. She completed a total of seven days of antibiotics. She was discharged home on PPI therapy and warfarin was restarted.
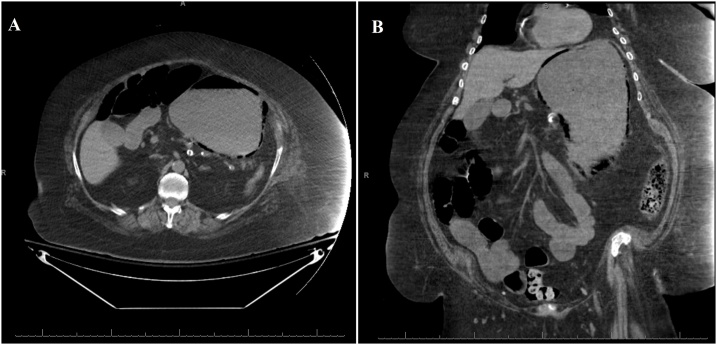
Non-contrast computed tomography scan of the abdomen revealed gastric distention and gastric pneumatosis throughout the wall of the stomach.
Computed tomography scan of the abdomen showing resolution of the gastric pneumatosis after 4 days.
2.2. Case 2
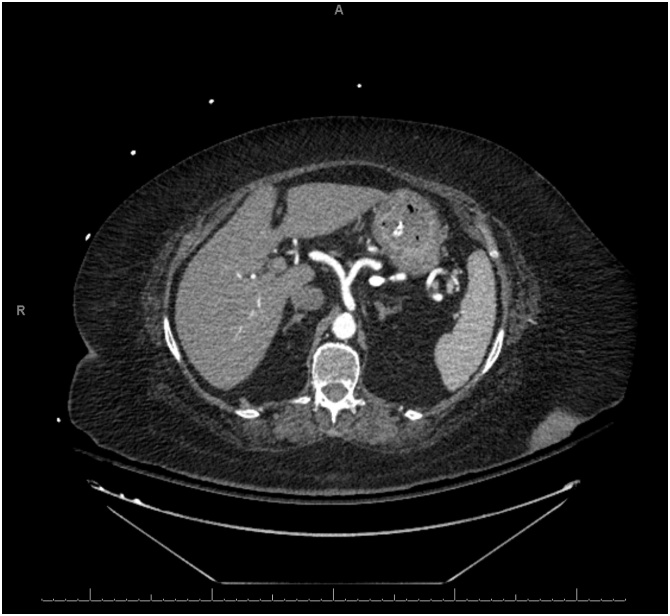
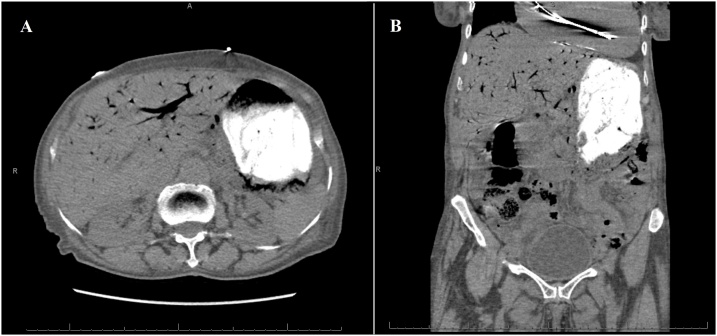
An 87-year-old presented to our emergency department with four days history of diffuse abdominal pain and associated non-bilious non-bloody emesis. She also complained of worsening shortness of breath and bilateral lower extremity edema over the last two weeks. She has a past medical history significant for congestive heart failure (CHF) with ejection fraction of 22%, pulmonary hypertension, and atrial fibrillation. She was on carvedilol and bumetanide. However, she has been non-compliant with her diuretic therapy. Her vital signs were significant for hypotension of 90/40. Abdominal exam was soft, non-distended, and non-tender without signs of peritonitis. Lung exam revealed clear lungs bilaterally without wheezing or crackles. She had bilateral lower extremity edema on exam. Laboratory studies showed a normal WBC of 7.0 cells/μL, creatinine of 1.04 mg/dL (baseline of 0.75 mg/dL), brain natriuretic peptide (BNP) of 868 pg/mL (reference <50 pg/mL), and lactate of 1.9 mmol/L. Electrocardiogram and serum troponin level were unremarkable. Chest roentgenogram revealed cardiomegaly with no evidence of pulmonary edema. CT scan of the abdomen without contrast revealed gastric pneumatosis with portal venous gas throughout the liver ( Fig. 3 ). The patient was admitted and restarted on her home bumetanide for possible CHF exacerbation. Her EG was managed non-operatively with PPI therapy, antibiotics (ceftriaxone and metronidazole), and NPO given her benign exam and hemodynamic stability. An EGD was not performed. Three days after admission, a repeat CT scan of the abdomen with contrast was obtained which showed resolution of the gastric pneumatosis and portal venous gas ( Fig. 4 ). Her pain has resolved at this time and she was started on an oral diet. After completing a total of seven days of antibiotics, she was discharged home on PPI therapy tolerating oral intake. The patient continues to do well two months after discharge.
Non-contrast computed tomography scan on presentation revealing gastric pneumotosis and extensive portal venous gas.
Computed tomography scan of the abdomen with contrast three days after the first scan showing resolution of the gastric pneumatosis and portal venous gas.
2.3. Case 3
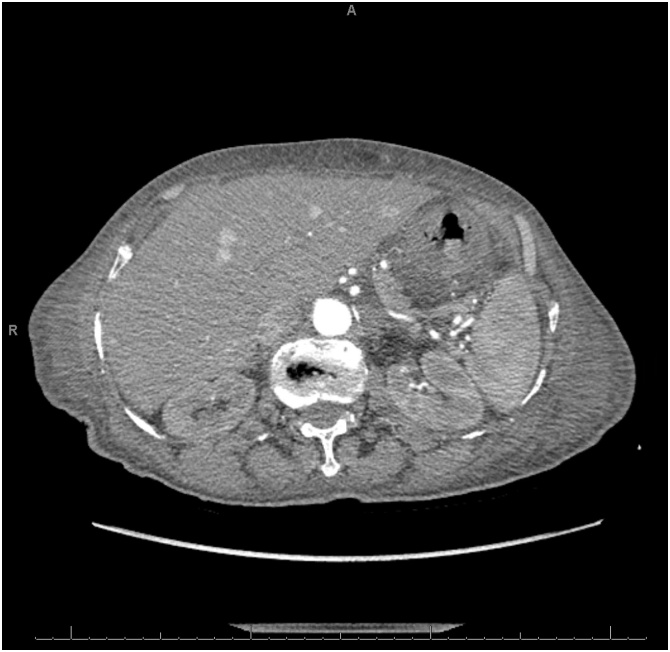
A 78-year-old female was brought into the emergency department from a long-term acute care facility for a chief complaint of mild diffuse abdominal pain over the last two days. She reported associated nausea and vomiting. Her past medical and surgical history were significant for bipolar disorder, congestive heart failure, hypertension, hypothyroidism, gastroesophageal reflux disease, and status post appendectomy. On arrival, her vital signs were significant for hypotension of 95/41. Her abdominal exam was soft, non-tender, and non-distended without signs of peritonitis. Her laboratory studies showed leukocytosis of 21,100 cells/μL and serum creatinine of 1.88 mg/dL (baseline of 0.7 mg/dL). CT scan of the abdomen with contrast revealed gastric pneumatosis with free intraperitoneal air ( Fig. 5 ). The patient was treated non-operatively with NGT decompression, NPO, intravenous fluid resuscitation, PPI, and antibiotics (piperacillin/tazobactam). An EGD was not performed. After three days, her pain gradually improved and her WBC and serum creatinine normalized. The NGT was removed and she was started on an oral diet. She was discharged back to her long-term acute care facility after completing a 1-week course piperacillin/tazobactam. The patient was admitted to another hospital two months later and expired from multi-organ failure from an unclear etiology.
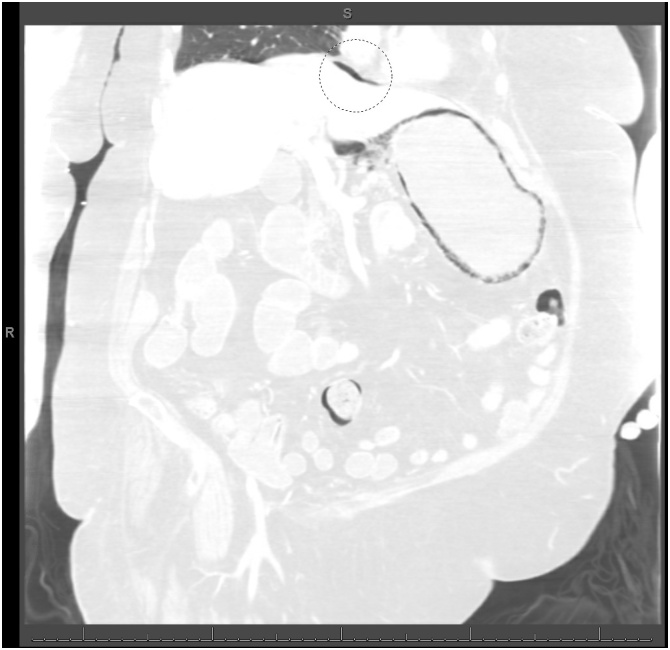
Computed tomography scan of the abdomen (lung window) showing extensive gastric pneumatosis and free intraperitoneal air superior to the liver (circle).
3. Discussion
EG is a rare but potentially fatal condition which is characterized by the presence of air in the wall of the stomach with associated systemic toxicity. Clinical presentation of EG is relatively non-specific and is typically characterized by abdominal pain, nausea, vomiting, and occasionally hematemesis. Physical examination findings vary depending on the degree of severity but can range from mild abdominal tenderness to peritonitis, especially in the setting of perforation. A distinction between EG and GE has been described in the literature. Since the two conditions have identical radiographic findings of gastric pneumatosis with an almost equal association with portal venous gas and pneumoperitoneum [ 1 ], we believe that the two entities likely represent various severities on the spectrum of the same disease. Distinction is primarily based on the severity of systemic toxicity and hemodynamic instability. Although the exact pathophysiology behind EG is not clearly understood, an ischemic injury to the gastric wall seems to be the inciting event for EG. This ischemic injury may lead to a secondary infection either from local bacterial invasion through the ulceration or from hematogenous spread [ 3 , 9 ]. In the cases we reported, only the second patient had a clear inciting event which was most likely an acute CHF exacerbation with a low flow state. The other two patients lacked a clear inciting event.
The diagnosis of EG is most commonly and best established on CT scan of the abdomen, although abdominal roentgenogram may be sufficient to make the diagnosis [ 3 , 7 ]. The extent of gastric emphysema as well as presence of portal venous gas and pneumoperitoneum in this setting do not correlate with the severity of disease or need for operative management [ 4 , 10 , 11 ]. Two of our reported cases had portal venous gas or pneumoperitoneum but did not require exploration given their relative stability, absence of peritoneal signs, and response to conservative management. An interesting finding that was noted in two of our cases that had a repeat CT scan in the first 3–4 days after diagnosis is the rapid resolution of gastric pneumatosis which coincided with improvement in symptoms. EGD usually identifies an inflamed, erosive, or necrotic area of mucosa in patient with EG [ 9 ]. The role of EGD in the diagnosis of EG has not been clearly defined despite its increased use in the management of EG in the last two decades [ 3 ]. Matsushima et al. recommended an EGD as part of the algorithm in the management of EG and the presence of ischemic gastric mucosa as an indication for surgical exploration [ 1 ]. However, Robinson et al. reported a case of EG with evidence of necrotic mucosa on EGD and portal venous gas on CT scan where non-operative management was chosen given hemodynamic stability with good outcome [ 4 ]. Alvin et al. described a case of a patient who underwent surgical exploration due to findings of severe erosive and necrotic gastritis on EGD without noting any evidence of gastric ischemia on subsequent exploration [ 12 ]. Thus, we believe that EGD findings are poor predictors for the presence of transmural ischemia and that operating solely on EGD findings of necrosis may lead to unnecessary extensive surgical interventions. On the other hand, EGD may have a role in patients who have clinical deterioration and surgical exploration is being considered [ 13 ]. EGD allows identification of the offending organism by culturing mucosal samples and allowing tailoring of antibiotic therapy accordingly. None of our patients underwent an EGD given clinical improvement with conservative management.
The management of EG initially includes intravenous fluid resuscitation, NPO, PPI, and broad-spectrum antibiotics covering gram negative and anaerobic organisms. The duration of antibiotic therapy is not well established, and we chose to only treat with 7 days as all three of our patients improved over the course of a few days. The addition of antifungal coverage may be necessary since Candida species is a possible infectious culprit. NGT decompression may be necessary especially in the setting of gastric distension on imaging, persistent emesis, and concern for bleeding. However, care must be taken as gastric perforation is a concern in this setting. Surgical exploration is indicated in patients who fail optimal medical management, demonstrate signs of clinical deterioration, and peritonitis [ 2 ]. According to a systematic review recently published by Watson et al., EG cases reported after the year 2000 were less likely to undergo surgical exploration (62.5% before 2000 versus 22.2% after 2000) with a lower associated mortality overall (59.4% before 2000 versus 33.3% after 2000) [ 3 ]. This reduction in mortality has been partially attributed to the lower rate of surgical intervention in the management of EG. Our approach is to utilize surgical exploration selectively and based on clinical deterioration regardless of CT scan findings with the utilization of EGD as an adjunct to help make the decision to operate in unclear cases.
4. Conclusion
EG is a rare condition presenting with findings of intramural gas in the stomach wall with associated signs of systemic toxicity. We present three cases of EG with various severities managed conservatively. Early recognition and the initiation of supportive care and antibiotics is key to prevent progression of this potentially fatal condition. The role of surgical intervention should be limited to cases where conservative therapy fails, or signs of peritonitis develop.
No source to be stated.
Ethical approval
The study is exempt from ethical approval in our institution.
Written informed consents were obtained for publication of two of the three cases and accompanying images. A copy of the written consents is available for review by the Editor-in-Chief of this journal on request. The third patient could not be reached despite exhaustive attempts and a letter attesting to that has been provided to the journal.
Registration of research studies
Not applicable.
Ann Woodward, MD.
Provenance and peer review
Not commissioned, externally peer-reviewed.
CRediT authorship contribution statement
Hassan Nasser: Writing - review & editing. Tommy Ivanics: Writing - original draft. Shravan Leonard-Murali: Visualization. Dania Shakaroun: Writing - review & editing. Ann Woodward: Supervision.
Declaration of Competing Interest
No conflicts of interest to be declared.
- 1. Matsushima K., Won E.J., Tangel M.R., Enomoto L.M., Avella D.M., Soybel D.I. Emphysematous gastritis and gastric emphysema: similar radiographic findings, distinct clinical entities. World J. Surg. 2015;39:1008–1017. doi: 10.1007/s00268-014-2882-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Yalamanchili M., Cady W. Emphysematous gastritis in a hemodialysis patient. South. Med. J. 2003;96:84–88. doi: 10.1097/01.SMJ.0000048085.35271.75. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Watson A., Bul V., Staudacher J., Carroll R., Yazici C. The predictors of mortality and secular changes in management strategies in emphysematous gastritis. Clin. Res. Hepatol. Gastroenterol. 2017;41:e1–e7. doi: 10.1016/j.clinre.2016.02.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Robinson S.L., Sadowski B.W., Eickhoff C., Mitre E., Young P.E. Emphysematous gastritis in a patient with untreated cyclic vomiting syndrome. ACG Case Rep. J. 2019;5:1–3. doi: 10.14309/crj.2018.90. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Van Mook W.N.K.A., Van der Geest S., Goessens M.L.M.J., Schoon E.J., Ramsay G. Gas within the wall of the stomach due to emphysematous gastritis: case report and review. Eur. J. Gastroenterol. Hepatol. 2002;14:1155–1160. doi: 10.1097/00042737-200210000-00018. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Ashfaq A., Chapital A.B. Emphysematous gastritis in a patient with coxsackie B3 myocarditis and cardiogenic shock requiring veno-arterial extra-corporeal membrane oxygenation. Int. J. Surg. Case Rep. 2015;14:121–124. doi: 10.1016/j.ijscr.2015.06.045. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Takano Y., Yamamura E., Gomi K., Tohata M., Endo T., Suzuki R., Hayashi M., Nakanishi T., Hanamura S., Asonuma K., Ino S., Kuroki Y., Maruoka N., Nagahama M., Inoue K., Takahashi H. Successful conservative treatment of emphysematous gastritis. Intern. Med. 2015;54:195–198. doi: 10.2169/internalmedicine.54.3337. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Agha R., Borrelli M., Farwana R., Koshy K., Fowler A., Orgill D. The PROCESS 2018 statement: Updating Consensus Preferred Reporting of CasE Series in Surgery (PROCESS) guidelines. Int. J. Surg. 2018;60:279–282. doi: 10.1016/j.ijsu.2018.10.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Rountree K.M., Lopez P.P. Emphysematous gastritis, a spectrum of disease: a four-case report. Am. Surg. 2017;83:E285–286. [ PubMed ] [ Google Scholar ]
- 10. Nehme F., Rowe K., Nassif I. Emphysematous gastritis with hepatic portal venous gas: a shift towards conservative management. BMJ Case Rep. 2017 doi: 10.1136/bcr-2017-219651. bcr-2017-219651. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Sharma P., Akl E.G. A combination of intramural stomach and portal venous air: conservative treatment. J. Commun. Hosp. Intern. Med. Perspect. 2016;6:1–4. doi: 10.3402/jchimp.v6.30519. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Alvin M., Al Jalbout N. Emphysematous gastritis secondary to Sarcina ventriculi. BMJ Case Rep. 2018 doi: 10.1136/bcr-2018-224233. bcr-2018-224233. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Nemakayala D.R., Rai M.P., Rayamajhi S., Jafri S.M. Role of conservative management in emphysematous gastritis. BMJ Case Rep. 2018;2018:2017–2019. doi: 10.1136/bcr-2017-222118. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.8 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Advertisement
Gastritis: An Update in 2020
- Stomach (P Malfertheiner, Section Editor)
- Open access
- Published: 26 August 2020
- Volume 18 , pages 488–503, ( 2020 )
Cite this article
You have full access to this open access article

- Massimo Rugge MD 1 , 2 ,
- Kentaro Sugano 3 ,
- Diana Sacchi 1 ,
- Marta Sbaraglia 1 &
- Peter Malfertheiner 4
46k Accesses
1 Altmetric
Explore all metrics
Purpose of review
The gastritis constellation includes heterogeneous clinicopathological entities, among which long-standing, non-self-limiting gastritis, mainly due to Helicobacter pylori infection, has been epidemiologically, biologically, and clinically linked to gastric cancer development (i.e. “inflammation-associated cancer”). This review illustrates the updated criteria applied in the taxonomy of gastritis (Kyoto classification), elucidates the biological rationale for endoscopy biopsy sampling (heterogeneity of gastric mucosa), and finally reports the results of long-term follow-up studies supporting the reliability of biopsy-based gastritis staging as predictor of gastritis-associated cancer risk.
Recent findings
By assuming gastric atrophy as the “cancerization field” where (non-syndromic) gastric cancer mostly develops, recent long-term follow-up studies consistently demonstrate the prognostic impact of the gastritis OLGA staging system.
Helicobacter pylori eradication is the leading strategy in the primary prevention of gastric cancer. In a multidisciplinary dimension of secondary cancer prevention, the OLGA staging system reliably ranks the patient-specific cancer risk, thus providing the clinical rationale for a tailored follow-up strategy.
Similar content being viewed by others
Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention

Current Perspectives in Atrophic Gastritis
Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication
Avoid common mistakes on your manuscript.
Introduction
The “gastritis” label is extensively (but inappropriately) applied to a spectrum of clinical symptoms relating to the upper abdomen, and the epigastrium in particular. The correct medical definition for this cluster of symptoms is dyspepsia. More strictly speaking, in the absence of organic disorders, the various combinations of upper digestive symptoms (e.g. bothersome postprandial fullness, early satiation, epigastric pain, and epigastric burning) should be defined as functional dyspepsia. There is an update on the (sub)types of functional dyspepsia at the Rome IV conference [ 1 , 2 ].
At endoscopy, gastritis is described as any reddening or edema of the gastric mucosa, but neither of these endoscopic features is specific or exclusive to mucosal inflammation. A Japanese study on the accuracy of standard endoscopic imaging for the detection of H. pylori infection reported accuracy of 89% for nodularity and 77% for mucosal swelling [ 3 ].
The endoscopic assessment of gastritis has advanced significantly in recent times thanks to the use of high-definition endoscopy and virtual chromoendoscopy (narrow-band imaging [NBI], blue light imaging [BLI], and linked color imaging [LCI]) [ 4 , 5 , 6 ]. An endoscopic classification of gastritis has been proposed in Japan, but important steps in the validation process are still needed before it can be more generally accepted outside Japan [ 7 ].
The endoscopic detection and interpretation of gastric atrophy poses the greatest challenge. Although the combined use of high-definition endoscopy and virtual chromoendoscopy has significantly improved the accuracy of endoscopic assessments, the consistent implementation of these new technologies entails a steep learning curve, and they are still operator-dependent [ 8 , 9 ].
In a recent study involving magnifying endoscopy combined with NBI, the authors reported that vascular architecture can detect premalignant gastric conditions accurately (88%), and better than the serum pepsinogen I/II ratio (74%) [ 10 ].
Gastritis: Classification criteria
From the clinical and pathological perspective, gastritis is defined as “acute” or “chronic.” These two terms are commonly intended as synonymous with self-limiting or non-self-limiting, respectively. The temporal parameter used to distinguish acute from chronic diseases is largely subjective, and many “chronic” asymptomatic diseases may occasionally develop “acute” symptoms.
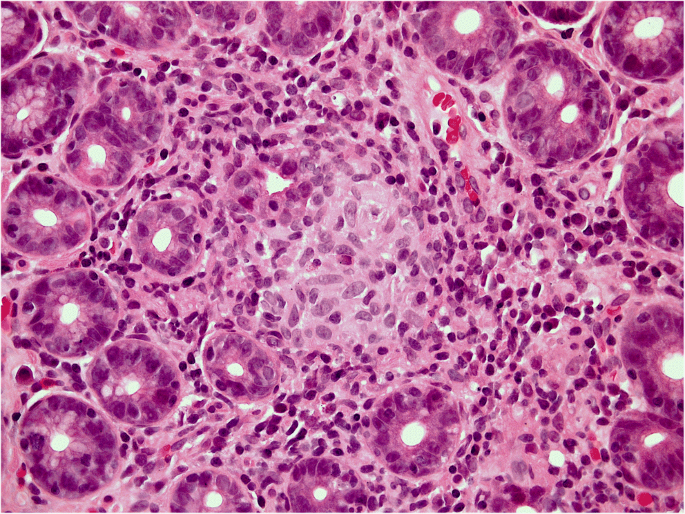
Equally questionable is the distinction between acute and chronic gastritis based on cellular inflammatory infiltrate: lymphocytes (typically associated with chronic inflammation) may prevail in some acute (i.e. self-limiting) forms of gastritis (lymphocytic gastritis), and granulocytes (typically associated with acute inflammatory lesions) can be found in chronic (i.e. non-self-limiting) inflammatory gastric diseases (e.g. H. pylori gastritis) (Figs. 1a, b and 2 ).
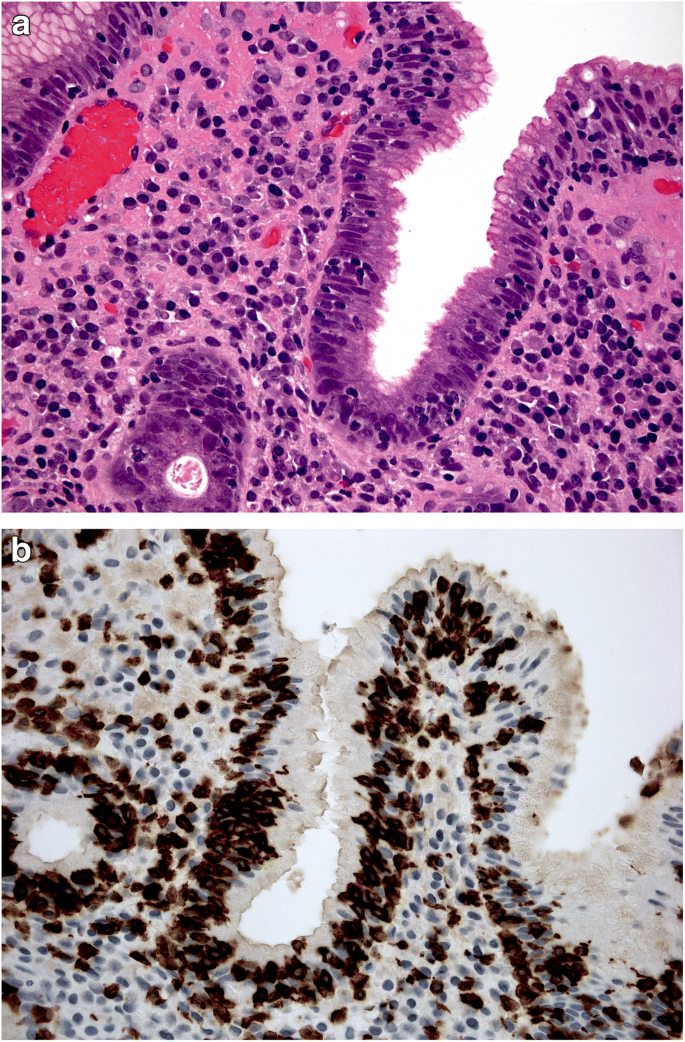
a , b Lymphocytic gastritis: high-grade mononuclear lymphocytic infiltrate within the lamina propria, in a gastric biopsy sample obtained from antral mucosa; intra-epithelial lymphocytes are also shown. ( a : Hematoxylin-eosin, original magnification ×40). In the same biopsy sample, the CD3-immunostain clearly documents the high-grade intra-epithelial lymphocytic infiltrate. ( b : CD3 immunostain, original magnification ×40).
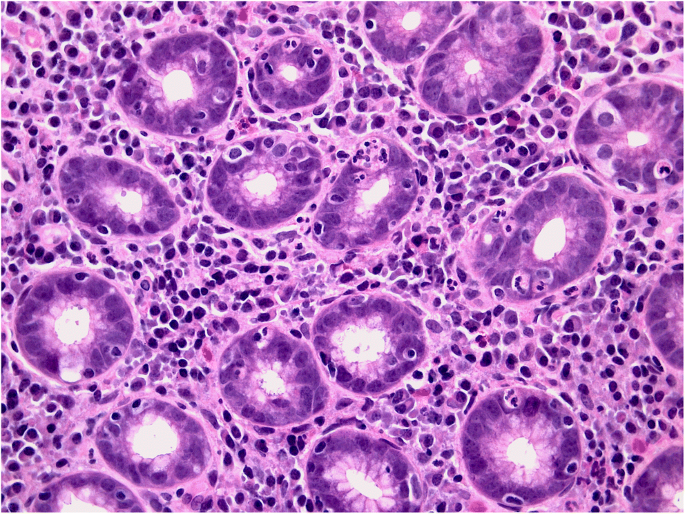
Helicobacter pylori gastritis: the picture shows the high-grade granulocytic infiltrate within the lamina propria and inside the gastric glans (so-called active inflammation). (Hematoxylin-eosin, original magnification ×40).
As recently acknowledged in the Kyoto global consensus report, the most valid gastritis classification relies on etiology. Leaving aside the (far from negligible) number of mucosal inflammations of unknown etiology, the agents involved in the etiology of gastritis can be gathered into two main groups: environmental agents (which may or may not be transmissible), and host-related, non-transmissible agents. The two etiopathogenic situations may overlap (Table 1 ). The etiological classification of gastritis (with minor adjustments to the Kyoto proposal) is shown in the table [ 11 ••, 12 , 13 , 14 ].
Excluding a false-negative H. pylori result remains a special challenge. For this purpose, marked reactive changes in both the antral and oxyntic mucosa need to be interpreted in the context of atrophy of other etiologies, or use of a proton pump inhibitor (PPI) [ 15 ]. El Zimaity et al. reported that H. pylori may only be found in parietal cells in patients on PPI. This is a rather exceptional situation and is not used as a criterion for assessing H. pylori -related gastritis. The differential diagnosis should include lymphocytic gastritis, vasculitis, granulomatous diseases, inflammatory bowel disease, viral infections, and other bacterial diseases. It is only after excluding all of these forms that the term “ H. pylori -negative gastritis” should be considered [ 15 , 16 , 17 , 18 , 19 , 20 •].
Gastritis: 2020 definition
By definition, a diagnosis of gastritis requires the histological detection of inflammatory cells within the lamina propria. Leukocytes may also penetrate the glandular lumen and/or spread down into the submucosa. The topography, severity, and cellularity of the inflammatory lesions might sometimes enable us to distinguish between different etiological variants of gastritis. Leaving aside the cellular profile of the “inflammatory population,” gastritis can be divided into two main forms, non-atrophic and atrophic. The need to distinguish between the two is crucially related to the different cancer risk associated with these two histological variants.
Non-atrophic gastritis exhibits mucosal damage that can even be extensive and/or severe, and may recover with trivial residual sequelae, or progress to atrophic phenotypes.
Atrophic gastritis is usually long-standing, and not self-limiting, and results from noxious agents that markedly alter the resident population of the gastric glands (i.e. oxyntic glands in the corpus/fundus, mucosecreting glands in the antral mucosa). In both these mucosal compartments, atrophy is defined as a “loss of native glandular units” [ 21 , 22 ].
Atrophy may result from a loss of native glands, replaced by fibrosis (non-metaplastic atrophy), or a focal or extensive replacement of the native gland population by metaplastic glands. Metaplastic atrophy may feature two phenotypes: intestinal metaplasia (IM), affecting the mucosecreting glands (Fig. 3 ), and pseudo-pyloric metaplasia, which is also known as pseudo-pyloric gland metaplasia (PGM) or spasmolytic peptide-expressing mucosa (SPEM), which only affects the oxyntic glands [ 23 , 24 ].
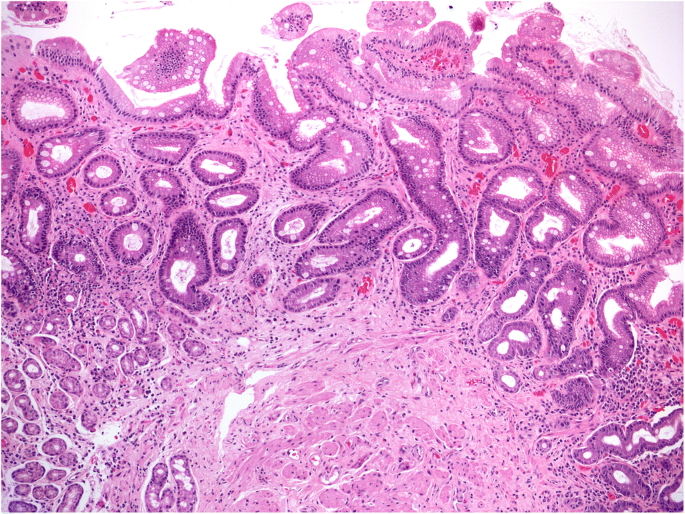
Gastric mucosa atrophy: metaplastic variant. The largest portion of the biopsy sample (about 65%; OLGA score: 3) is covered by atrophic-metaplastic glands; goblet cells are the key marker of mucosal intestinalization. (Hematoxylin-eosin, original magnification ×5)
The histogenesis of SPEM is still debated and needs to be further addressed. Seminal experiments supported the hypothesis that SPEM originates (independently of stem cells) from “transdifferentiation” or “dedifferentiation” [ 25 , 26 ]. Other putative sources [ 27 , 28 ] include the progenitor cells located at the glandular neck, or a population of Troy-positive cells located at the bottom of the oxyntic glands. The idea of a possible transformation of SPEM into intestinalized epithelia is supported by molecular profiling studies and histological evidence of SPEM preceding the histological detection of oxyntic intestinalization by years.
Both subtypes of metaplastic changes are included in the spectrum of gastric atrophy [ 29 , 30 ].
The inclusion of other (minor) variants of metaplastic transformation (e.g. pseudo-pancreatic metaplasia) in the spectrum of atrophy is irrelevant. Any subtype of gastric atrophy may give rise to functional changes (in acid secretion or pepsinogen serum levels, for instance) and/or a “remodeling” of the intragastric microenvironment. There is increasing evidence to suggest that the “atrophy-associated” microbiome may be involved in promoting a cancer-prone microenvironment. Such a picture reinforces the priority of assessing and quantifying gastric mucosal atrophy endoscopically and histologically, considering the whole spectrum of atrophy phenotypes, all of which might be involved in creating cancer-promoting biological conditions. Taking this new approach, we need to reconsider the “traditional” view concerning the specific responsibility of intestinal metaplasia in gastric carcinogenesis, and return to a holistic concept of atrophy as the “cancerization field” for gastric adenocarcinoma [ 24 , 31 , 32 , 33 , 34 , 35 , 36 ].
Gastritis: Biopsy sampling protocols and histological assessments
The rationale behind gastric biopsy sampling takes into account the physiopathological compartmentalization of the gastric mucosa. Already in embryo, the gastric sac consists of two (functional, histological) compartments, and they remain distinct after birth.
Gastric compartmentalization (from the embryo to the adult)
There is a paucity of (sometimes contradictory) information on the characterization of the embryonic stomach: canonical Wnt signaling pathway is assumed to be virtually restricted to the cranial gastric segment and absent from the presumptive antrum. The distinctive profile of the embryonic stomach is supported by two recent complementary observations [ 37 , 38 ]: the deletion of Ctnnb1 , a canonical WNT-effector, is associated with ectopic Pdx1 expression, which results in cranial stomach antralization; and the deletion of Bapx1 (a transcription factor expressed in the mesenchyme of the distal stomach) results in an “oxyntic transformation” of the antral stomach (Fig. 4 ).
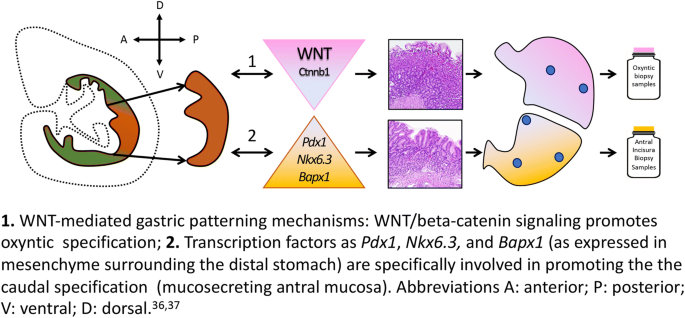
1. WNT-mediated gastric patterning mechanisms: WNT/beta-catenin signaling promotes oxyntic specification. 2. Transcription factors such as Pdx1, Nkx6.3, and Bapx1 (as expressed in mesenchyme surrounding the distal stomach) are specifically involved in promoting the caudal specification (mucosecreting antral mucosa). Abbreviations A: anterior; P: posterior; V: ventral; D: dorsal [ 36 , 37 ]
At birth, there are two main, histologically distinct gastric mucosal compartments: the oxyntic (corpus) mucosa, and the mucosecreting (antral) mucosa. A third area covers a narrow portion of the junctional area between the terminal esophagus and the most cranial segment of the stomach. The phenotype of native cardia-mucosa is still controversial. Equally credited theories support a glandular population including both native mucosecreting (antral type) and native oxyntic glands, or a mucosa natively consisting only of native oxyntic glands, with any mucosecreting component considered a metaplastic transformation of the resident oxyntic glands.
Distally to the cardia, the largest portion of the gastric mucosa consists of oxyntic glands (including chief, parietal end enterochromaffin-like [ECL] cells). The so-called atrophic border, better defined as the pyloric-fundic gland border, is an irregular boundary between the oxyntic and the mucosecreting compartments. The latter compartment consists of coiled mucosecreting glands extending from approximately two-thirds of the length of the stomach hook to the pyloric sphincter. The normal morphology of these areas is well established and easily recognizable. All these anatomical landmarks have important implications in the endoscopic and histological assessment of gastritis.
Obtaining gastric mucosa biopsy samples: The biological rationale
The different embryonic “patterning” and the post-natal histology and function of the stomach lend further support to the view of this organ as “two functions in one sac.” This compartmentalization provides the rationale for obtaining gastric biopsy samples representative of both mucosal compartments. In routine endoscopic practice, however, this recommendation is frequently ignored, for two main reasons: the increasing costs of the diagnostic procedure associated with obtaining, handling, and assessing multiple biopsy samples; and a preference for using one (or even more!) biopsy samples for a rapid assessment of Helicobacter pylori status.
Different protocols for the endoscopic biopsy sampling of the gastric mucosa have been proposed, but they all recommend obtaining samples from both the oxyntic and the mucosecreting mucosa. The recommended number of specimens to collect varies, depending on the clinical indication, and the aims of the endoscopy/biopsy procedure. In general, more extensive biopsy protocols are recommended for research purposes, while a minimum set of gastric biopsies is suggested for clinical purposes. The most widely applied clinical protocol is the updated Sydney System, which includes a total of five biopsy specimens, i.e. two from the oxyntic mucosa (both submitted in the same vial), one from the incisura angularis, and two from the antral mucosa (all three submitted in the same vial). Any focal lesions should also be sampled and submitted separately [ 8 , 39 , 40 •, 41 ].
Gastritis: The clinical value of a “structured” histology report
Gastric mucosal atrophy is recognized as the cancerization field in which more than 90% of gastric epithelial malignancies will eventually develop. This well-established evidence provides the rationale for assessing patients’ “individual” gastric cancer risk from their histological atrophy score, which also takes its topography (corpus, antrum, or both) into account. Consistent with this approach, histological gastritis staging has proved a reliable predictor of gastric cancer risk.
In East Asian countries, the extent of atrophy and its associated cancer risk continues to be assessed endoscopically according to the Kimura-Takemoto classification [ 42 , 43 , 44 ]. In the Western world, a biopsy-based assessment is recommended (Maastricht V, MAPS guidelines). OLGA (Operative Link for Gastritis Assessment) is a validated staging system that considers both the atrophy score and the compartment (oxyntic versus mucosecreting) from which the biopsy samples were obtained (i.e. oxyntic and antral mucosa, according to the Sydney System protocol) [ 45 , 46 ]. The combined antral and oxyntic scores (see Table 2 for atrophy scoring and staging) result in a “gastritis stage” (from 0 to IV) indicating the risk of malignancy. An alternative staging system called OLGIM (Operative Link on Gastric Intestinal Metaplasia) is based only on a score for intestinal metaplasia [ 47 , 48 ], and this makes it likely to be less sensitive in assessing atrophy than the (more comprehensive) OLGA approach [ 48 ].
Whenever possible, histological staging should be complemented with etiological information, reference to clinical parameters, and other diagnostic details. Table 1 lists all the relevant etiological factors, and those most often resulting in atrophic mucosal changes.
From the histology report to the clinical patient’s management
In spite of the enormous progress made in endoscopic imaging technologies, with more to come, histology remains the gold standard for the diagnosis of gastritis. Advances in this area relate to an etiology-based diagnosis, already reflected in the new International Classification of Diseases (ICD-11), and to the clinical priority of gastritis staging in the long-term personalized management of gastritis patients. Relying on essential information about the etiology and staging of a case of gastritis, clinicians can safely proceed with the most appropriate therapy, and decide whether and what type of follow-up is required [ 49 , 50 ].
Having identified the cause of gastritis, the first step will be to eliminate the noxious agent whenever possible. In cases of H. pylori gastritis, eradication therapy is recommended in all international consensus and guideline reports [ 11 ••, 51 , 52 ].
In the absence of severe gastric disease and/or preneoplastic changes, the success of H. pylori eradication therapy can be tested with a noninvasive surrogate marker (urea breath test [UBT], fecal antigen test). A negative test result indicates the healing of H. pylori gastritis [ 53 ••]. Any specific infectious agent detected can be cured by appropriate antibiotic or antiviral treatments. No etiological therapy is available as yet for autoimmune gastritis, the clinical impact of which is constantly increasing all over the industrialized world. There is little evidence for glucocorticoid therapy to reverse the atrophy involved [ 54 ]. When pernicious anemia ensues, vitamin B12 supplementation is indicated. In the autoimmune setting, the (consistently recognized) risk of neuroendocrine neoplasia makes it important to adopt an appropriate staging system that includes IM-negative atrophic transformation (i.e. pseudo-pyloric metaplasia, or what is known as SPEM) [ 55 , 56 ]. SPEM is involved in hyperplastic/dysplastic ECL cell proliferation, and may theoretically be associated with a higher risk of gastric adenocarcinoma [ 25 , 57 , 58 ]. It is often more difficult to document the etiology of other forms of gastritis, but it may help to look for a systemic cause, as in Crohn’s disease (Fig. 5 ).
Epithelioid granuloma in Crohn-associated gastritis. Infectious etiology was excluded by both molecular biology testing and special stain for Koch’s bacilli. (Hematoxylin-eosin, original magnification ×40)
Leaving aside the etiology of gastritis, a histology report plainly expressing the “level of alarm” related to the severity of atrophic disease (and its associated cancer risk, in particular) could contribute to generating treatment and follow-up protocols tailored to individual patients’ clinical needs. This approach has been extensively applied in oncological practice and is the reasoning on which gastritis staging is based. In patients with “advanced” atrophic changes (high-risk OLGA/OLGIM stages III/IV), regular endoscopic follow-up serves the fundamental purpose of a reliable secondary gastric cancer prevention strategy [ 59 ].
Three different trials with a follow-up spanning several years produced significant evidence to support a high risk of gastric cancer developing in patients with OLGA stages III/IV (Table 3 ) [ 40 •, 60 , 61 ••]. Two of these three studies [ 40 •, 61 ••] were conducted in populations of consecutive dyspeptic patients undergoing endoscopy, with no histological evidence of neoplastic lesions (intraepithelial [IEN] or invasive gastric cancer [GC]). The studies had a similar patient distribution by stage, and the proportion of patients in stages III and IV never exceeded 3% (these patients were mainly males, and their median age was older than that of patients with stage 0/I/II disease). Such a low prevalence of patients “at risk” makes it feasible to implement dedicated programs of active surveillance. The two studies (involving 9191 patients in all) also consistently showed a dichotomous cancer risk, which was nil or negligible for stages 0/I/ II (low-risk stages) and high for OLGA stages III/IV (high-risk stages).
The third study [ 60 ] involved 93 patients who were followed up with paired endoscopies for more than 12 years (range: 144–204 months). It was conducted on a population living in a mountain valley previously identified as being at high risk of GC (with an incidence of gastric malignancy of 270/100,000/5 years, and an 80% prevalence of H. pylori ). As expected in this particular epidemiological context, the prevalence of high-risk stages was greater than in the two previously mentioned studies, but all the incident neoplastic lesions (detected by the end of the follow-up) were identified among patients enrolled with stage III/IV gastritis.
The high risk of cancer developing in such cases enables us to identify patients early enough in their progression to gastric malignancy for timely intervention, which involves endoscopic resection of crucial areas of the stomach showing signs of early gastric cancer. Further adjunctive evidence significantly links high-risk OLGA gastritis with H. pylori status, but eradication therapies may be ineffective in the more advanced stages of gastritis (particularly when it is already associated with intraepithelial neoplastic lesions, with male gender, or with advanced age) [ 41 , 62 , 63 ].
Histological staging should be combined with other information (family history, serology, endoscopy) to enable follow-up schedules to be tailored to a given patient’s clinical and pathological profile [ 64 ]. Even in patients with early gastric cancer, further progression can be stopped by adequate management of their gastritis, as shown in the Korean study by Choi [ 65 ].
As mentioned earlier, gastric (atrophic) intestinalization only accounts for one of the three “faces” of the atrophic transformation of the mucosa. Restricting the atrophy score to IM alone (as in the OLGIM approach) can have significant drawbacks in terms of a conclusive staging of gastritis, ultimately excluding patients who warrant a “dedicated” secondary prevention strategy from an appropriate follow-up program [ 13 ]. The updated endoscopic guidelines for managing gastritis fully embrace recent progress and the clinical relevance of proper gastritis staging for the purpose of cancer prevention [ 42 ].
Outlook into 2020
According to the Kyoto Global Consensus Conference, etiology is taken for reference in the classification of gastritis. The etiological picture of long-standing gastritis can include both environmental (e.g. H. pylori ) and host-related (e.g. Autoimmunity) agents, potentially resulting in the atrophic transformation of native gastric mucosa. Epidemiological evidence implicates the atrophic microenvironment in H. pylori gastritis as a major factor responsible for the etiopathogenesis of more than 90% of gastric malignancies.
The atrophic transformation of gastric mucosa gives rise to different histological phenotypes, all of which have been biologically profiled and can be histologically scored. They may also be associated with a range of functional changes, which can serve as (quantitative) serological markers of the atrophic process. It is easy to imagine the atrophy-remodeled gastric microbiota having a role as co-promoter in the atrophic cancer-prone microenvironment.
In Western countries, histology-based gastritis staging (with the OLGA system) has consistently proved reliable in predicting the cancer risk associated with atrophy. Any (complementary) endoscopy-based risk assessment (as suggested in authoritative literature) is also welcome. The reliability of endoscopic staging still needs to be supported by large-scale studies, however, and validated in terms of its clinical reproducibility.
Over the coming years, we will see how this multidisciplinary approach can be optimized for the purpose of designing global strategies for eradicating gastric cancer and implementing patient-tailored prevention strategies. The available evidence does suggest that combining primary and secondary prevention strategies can realistically succeed in cutting the epidemiological impact of gastric cancer – the world’s fourth leading cause of cancer-related death.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • of importance •• of major importance.
Talley NJ. Editorial: moving away from focussing on gastric pathophysiology in functional dyspepsia: new insights and therapeutic implications. Am J Gastroenterol. 2017;112(1):141–4. https://doi.org/10.1038/ajg.2016.519 .
Article PubMed Google Scholar
Miwa H, Oshima T, Tomita T, et al. Recent understanding of the pathophysiology of functional dyspepsia: role of the duodenum as the pathogenic center. J Gastroenterol. 2019;54(4):305–11. https://doi.org/10.1007/s00535-019-01550-4 .
Article PubMed PubMed Central Google Scholar
Okamura T, Iwaya Y, Kitahara K, Suga T, Tanaka E. Accuracy of endoscopic diagnosis for mild atrophic gastritis infected with Helicobacter pylori . Clin Endosc. 2018;51(4):362–7. https://doi.org/10.5946/ce.2017.177 .
Fukuda H, Miura Y, Osawa H, et al. Linked color imaging can enhance recognition of early gastric cancer by high color contrast to surrounding gastric intestinal metaplasia. J Gastroenterol. 2019;54(5):396–406. https://doi.org/10.1007/s00535-018-1515-6 .
Article CAS PubMed Google Scholar
Takeda T, Asaoka D, Nojiri S, et al. Linked color imaging and the Kyoto classification of gastritis: evaluation of visibility and inter-Rater reliability. Digestion. 2019:1–10. https://doi.org/10.1159/000501534 .
Sun X, Bi Y, Nong B, et al. Linked color imaging confers benefits in profiling H. pylori infection in the stomach. Endosc Int open. 2019;7(7):E885–92. https://doi.org/10.1055/a-0895-5377 .
Dohi O, Majima A, Naito Y, et al. Can image-enhanced endoscopy improve the diagnosis of Kyoto classification of gastritis in the clinical setting? Dig Endosc. 2019:den.13540. https://doi.org/10.1111/den.13540 .
Rugge M, Fassan M, Tsukanov VV, Meggio A, de Boni M. From open-type atrophic gastritis to gastritis staging. Dig Dis Sci. 2011;56(6):1917–8. https://doi.org/10.1007/s10620-011-1705-z .
Mizukami K, Ogawa R, Okamoto K, et al. Objective endoscopic analysis with linked color imaging regarding gastric mucosal atrophy: a pilot study. Gastroenterol Res Pract. 2017;2017:5054237. https://doi.org/10.1155/2017/5054237 .
White JR, Sami SS, Reddiar D, et al. Narrow band imaging and serology in the assessment of premalignant gastric pathology. Scand J Gastroenterol. 2018;53(12):1611–8. https://doi.org/10.1080/00365521.2018.1542455 .
•• Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–67. https://doi.org/10.1136/gutjnl-2015-309252 . The publication refers the results of a global consensus concerning the taxonomy of gastritis.
Mahachai V, Vilaichone R-K, Pittayanon R, et al. Helicobacter pylori management in ASEAN: the Bangkok consensus report. J Gastroenterol Hepatol. 2018;33(1):37–56. https://doi.org/10.1111/jgh.13911 .
Rugge M, Sugano K, Scarpignato C, Sacchi D, Oblitas WJ, Naccarato AG. Gastric cancer prevention targeted on risk assessment: gastritis OLGA staging. Helicobacter. 2019;24(2):e12571. https://doi.org/10.1111/hel.12571 .
Hunt RH, Camilleri M, Crowe SE, et al. The stomach in health and disease. Gut. 2015;64(10):1650–68. https://doi.org/10.1136/gutjnl-2014-307595 .
El-Zimaity H, Choi W-T, Lauwers GY, Riddell R. The differential diagnosis of Helicobacter pylori negative gastritis. Virchows Arch. 2018;473(5):533–50. https://doi.org/10.1007/s00428-018-2454-6 .
Choi W-T, Lauwers GY. Patterns of gastric injury: beyond Helicobacter pylori . Surg Pathol Clin. 2017;10(4):801–22. https://doi.org/10.1016/j.path.2017.07.003 .
Weinstein R, Riordan MA, Wenc K, Kreczko S, Zhou M, Dainiak N. Dual role of fibronectin in hematopoietic differentiation. Blood. 1989;73(1):111–6. http://www.ncbi.nlm.nih.gov/pubmed/2910353 . Accessed 19 Nov 2019.
Nordenstedt H, Graham DY, Kramer JR, et al. Helicobacter pylori -negative gastritis: prevalence and risk factors. Am J Gastroenterol. 2013;108(1):65–71. https://doi.org/10.1038/ajg.2012.372 .
Shiota S, Thrift AP, Green L, et al. Clinical manifestations of Helicobacter pylori -negative gastritis. Clin Gastroenterol Hepatol. 2017;15(7):1037–1046.e3. https://doi.org/10.1016/j.cgh.2017.01.006 .
• Rugge M, Genta RM, Di Mario F, et al. Gastric cancer as preventable disease. Clin Gastroenterol Hepatol. 2017;15(12):1833–43. https://doi.org/10.1016/j.cgh.2017.05.023 . This comprehensive review provides the biological and clinical rationale for gastric cancer prevention strategies.
Ruiz B, Garay J, Johnson W, et al. Morphometric assessment of gastric antral atrophy: comparison with visual evaluation. Histopathology. 2001;39(3):235–42. https://doi.org/10.1046/j.1365-2559.2001.01221.x .
Rugge M, Correa P, Dixon MF, et al. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther. 2002;16(7):1249–59. https://doi.org/10.1046/j.1365-2036.2002.01301.x .
Zambon C-F, Basso D, Navaglia F, et al. Helicobacter pylori virulence genes and host IL-1RN and IL-1beta genes interplay in favouring the development of peptic ulcer and intestinal metaplasia. Cytokine. 2002;18(5):242–51. https://doi.org/10.1006/cyto.2002.0891 .
Graham DY, Rugge M, Genta RM. Diagnosis: gastric intestinal metaplasia – what to do next? Curr Opin Gastroenterol. 2019;35(6):535–43. https://doi.org/10.1097/MOG.0000000000000576 .
Article CAS PubMed PubMed Central Google Scholar
Goldenring JR. Pyloric metaplasia, pseudopyloric metaplasia, ulcer-associated cell lineage and spasmolytic polypeptide-expressing metaplasia: reparative lineages in the gastrointestinal mucosa. J Pathol. 2018;245(2):132–7. https://doi.org/10.1002/path.5066 .
Radyk MD, Burclaff J, Willet SG, Mills JC. Metaplastic cells in the stomach arise, independently of stem cells, via dedifferentiation or transdifferentiation of chief cells. Gastroenterology. 2018;154(4):839–843.e2. https://doi.org/10.1053/j.gastro.2017.11.278 .
Hayakawa Y, Ariyama H, Stancikova J, et al. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28(6):800–14. https://doi.org/10.1016/j.ccell.2015.10.003 .
Hayakawa Y, Fox JG, Wang TC. Isthmus stem cells are the origins of metaplasia in the gastric corpus. Cell Mol Gastroenterol Hepatol. 2017;4(1):89–94. https://doi.org/10.1016/j.jcmgh.2017.02.009 .
Rugge M, Pennelli G, Pilozzi E, et al. Gastritis: the histology report. Dig Liver Dis. 2011;43(Suppl 4):S373–84. https://doi.org/10.1016/S1590-8658(11)60593-8 .
Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40(8):650–8. https://doi.org/10.1016/j.dld.2008.02.030 .
Parsons BN, Ijaz UZ, D’Amore R, et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori -induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017;13(11):e1006653. https://doi.org/10.1371/journal.ppat.1006653 .
Badal VD, Wright D, Katsis Y, et al. Challenges in the construction of knowledge bases for human microbiome-disease associations. Microbiome. 2019;7(1):129. https://doi.org/10.1186/s40168-019-0742-2 .
Park CH, Lee A-R, Lee Y-R, Eun CS, Lee SK, Han DS. Evaluation of gastric microbiome and metagenomic function in patients with intestinal metaplasia using 16S rRNA gene sequencing. Helicobacter. 2019;24(1):e12547. https://doi.org/10.1111/hel.12547 .
Del Prete A, Allavena P, Santoro G, Fumarulo R, Corsi MM, Mantovani A. Molecular pathways in cancer-related inflammation. Biochem Medica. 2011:264–75. https://doi.org/10.11613/BM.2011.036 .
Iino C, Shimoyama T, Chinda D, Sakuraba H, Fukuda S, Nakaji S. Influence of Helicobacter pylori infection and atrophic gastritis on the gut microbiota in a Japanese population. Digestion. 2019:1–11. https://doi.org/10.1159/000500634 .
McDonald SAC, Greaves LC, Gutierrez-Gonzalez L, et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134(2):500–10. https://doi.org/10.1053/j.gastro.2007.11.035 .
McCracken KW, Wells JM. Mechanisms of embryonic stomach development. Semin Cell Dev Biol. 2017;66:36–42. https://doi.org/10.1016/j.semcdb.2017.02.004 .
Thompson CA, DeLaForest A, Battle MA. Patterning the gastrointestinal epithelium to confer regional-specific functions. Dev Biol. 2018;435(2):97–108. https://doi.org/10.1016/j.ydbio.2018.01.006 .
Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30. https://doi.org/10.1136/gutjnl-2016-312288 .
• Rugge M, Genta RM, Fassan M, et al. OLGA gastritis staging for the prediction of gastric cancer risk: a long-term follow-up study of 7436 patients. Am J Gastroenterol. 2018;113(11):1621–8. https://doi.org/10.1038/s41395-018-0353-8 . In this long-term follow-up study of 7436 patients, a significant increased risk of gastric cancer was only associated with patients harbouring OLGA stages III/IV at their enrollment.
Rugge M, Sacchi D, Graham DY, Genta RM. Secondary prevention of gastric cancer: merging the endoscopic atrophic border with OLGA staging. Gut. 2019:gutjnl-2019-319107. https://doi.org/10.1136/gutjnl-2019-319107 .
Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51(4):365–88. https://doi.org/10.1055/a-0859-1883 .
Kono S, Gotoda T, Yoshida S, et al. Can endoscopic atrophy predict histological atrophy? Historical study in United Kingdom and Japan. World J Gastroenterol. 2015;21(46):13113–23. https://doi.org/10.3748/wjg.v21.i46.13113 .
Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1(03):87–97. https://doi.org/10.1055/s-0028-1098086 .
Article Google Scholar
Price AB. The Sydney system: histological division. J Gastroenterol Hepatol. 1991;6(3):209–22. https://doi.org/10.1111/j.1440-1746.1991.tb01468.x .
Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56(5):631–6. https://doi.org/10.1136/gut.2006.106666 .
Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71(7):1150–8. https://doi.org/10.1016/j.gie.2009.12.029 .
Rugge M, Fassan M, Pizzi M, et al. Operative link for gastritis assessment vs operative link on intestinal metaplasia assessment. World J Gastroenterol. 2011;17(41):4596–601. https://doi.org/10.3748/wjg.v17.i41.4596 .
Meggio A, Mariotti G, Gentilini M, de Pretis G. Priority and appropriateness of upper endoscopy out-patient referrals: two-period comparison in an open-access unit. Dig Liver Dis. 2019;51(11):1562–6. https://doi.org/10.1016/j.dld.2019.05.028 .
Teriaky A, AlNasser A, McLean C, Gregor J, Yan B. The utility of endoscopic biopsies in patients with normal upper endoscopy. Can J Gastroenterol Hepatol. 2016;2016:3026563. https://doi.org/10.1155/2016/3026563 .
Schulz C, Schütte K, Koch N, et al. The active bacterial assemblages of the upper GI tract in individuals with and without Helicobacter infection. Gut. 2018;67(2):216–25. https://doi.org/10.1136/gutjnl-2016-312904 .
Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology. 2019;157(1):44–53. https://doi.org/10.1053/j.gastro.2019.04.011 .
•• Malfertheiner P. Diagnostic methods for H. pylori infection: choices, opportunities and pitfalls. United Eur Gastroenterol J. 2015;3(5):429–31. https://doi.org/10.1177/2050640615600968 . The review critically considers the different (non-invasive and invasive) methods clinically applied in the assessment of current Helicobacer pylori infection.
Article CAS Google Scholar
Jeffries GH, Todd JE, Sleisenger MH. The effect of prednisolone on gastric mucosal histology, gastric secretion, and vitamin B 12 absorption in patients with pernicious anemia. J Clin Invest. 1966;45(5):803–12. https://doi.org/10.1172/JCI105395 .
Zhang M, Liu S, Hu Y, et al. Biopsy strategies for endoscopic screening of pre-malignant gastric lesions. Sci Rep. 2019;9(1):14909. https://doi.org/10.1038/s41598-019-51487-0 .
Isajevs S, Liepniece-Karele I, Janciauskas D, et al. Gastritis staging: interobserver agreement by applying OLGA and OLGIM systems. Virchows Arch. 2014;464(4):403–7. https://doi.org/10.1007/s00428-014-1544-3 .
Halldórsdóttir AM, Sigurdardóttrir M, Jónasson JG, et al. Spasmolytic polypeptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci. 2003;48(3):431–41. https://doi.org/10.1023/a:1022564027468 .
Koulis A, Buckle A, Boussioutas A. Premalignant lesions and gastric cancer: current understanding. World J Gastrointest Oncol. 2019;11(9):665–78. https://doi.org/10.4251/wjgo.v11.i9.665 .
Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology . 2019:his.13975. https://doi.org/10.1111/his.13975 .
Rugge M, de Boni M, Pennelli G, et al. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Aliment Pharmacol Ther. 2010;31(10):1104–11. https://doi.org/10.1111/j.1365-2036.2010.04277.x .
•• Rugge M, Meggio A, Pravadelli C, et al. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: a 5-year prospective study of 1755 patients. Gut. 2019;68(1):11–7. https://doi.org/10.1136/gutjnl-2017-314600 . In a large cohort of 1755 dyspeptic outpatients undergoing upper gastrointestinal endoscopy, this long-term follow-up study provides evidence that a significant risk of neoplastic progression is only associated with patients harboring OLGA stages III/IV at their enrollment.
de Vries AC, Kuipers EJ, Rauws EAJ. Helicobacter pylori eradication and gastric cancer: when is the horse out of the barn? Am J Gastroenterol. 2009;104(6):1342–5. https://doi.org/10.1038/ajg.2008.15 .
Rugge M, de Boni M, Pennelli G, Mescoli C, Graham DY. OLGA can guard the barn. Am J Gastroenterol. 2009;104(12):3099. author reply 3101-2. https://doi.org/10.1038/ajg.2009.512 .
Genta RM, Rugge M. Gastric precancerous lesions: heading for an international consensus. Gut. 1999;45(Suppl 1):I5–8. http://www.ncbi.nlm.nih.gov/pubmed/10457028 .
Choi IJ, Kim Y-I, Park B. Helicobacter pylori and prevention of gastric cancer. N Engl J Med. 2018;378(23):2244–5. https://doi.org/10.1056/NEJMc1805129 .
Download references
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. This work was partly supported by grants from the Italian Association for Cancer Research (AIRC Regional grant no. 6421 to MR).
Author information
Authors and affiliations.
Department of Medicine (DIMED), Surgical Pathology & Cytopathology Unit, University of Padua, Via A. Gabelli, 61, 35121, Padova, Italy
Massimo Rugge MD, Diana Sacchi & Marta Sbaraglia
Veneto Tumor Registry (RTV), Veneto Regional Authority, Padova, Italy
Massimo Rugge MD
Department of Medicine, Jichi Medical University, Tochigi, Japan
Kentaro Sugano
Department of Internal Medicine II, Hospital of the Ludwig Maximilian University of Munich, Munich, Germany
Peter Malfertheiner
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Massimo Rugge MD .
Ethics declarations
Conflict of interest.
Massimo Rugge, Kentaro Sugano, Diana Sacchi, Marta Sbaraglia, and Peter Malfertheiner declare no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stomach
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Rugge, M., Sugano, K., Sacchi, D. et al. Gastritis: An Update in 2020. Curr Treat Options Gastro 18 , 488–503 (2020). https://doi.org/10.1007/s11938-020-00298-8
Download citation
Published : 26 August 2020
Issue Date : September 2020
DOI : https://doi.org/10.1007/s11938-020-00298-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Atrophic gastritis
- OLGA staging
- Gastric oncogenesis
- Intestinal metaplasia
- Helicobacter pylori
- Spasmolytic peptide expressing mucosa
- Find a journal
- Publish with us
- Track your research
CASE REPORT article
Case report: rapid onset, ischemic-type gastritis after initiating oral iron supplementation.

- 1 Department of Medicine, University of Illinois College of Medicine, Chicago, IL, United States
- 2 Medical Scientist Training Program, University of Illinois College of Medicine, Chicago, IL, United States
Oral iron supplements are commonly administered to patients with chronic iron deficiency anemia. This approach is generally well-tolerated, causing only mild adverse effects. Rarely, oral iron supplementation can cause more severe symptoms, one of the most concerning being acute gastritis. This predominantly affects elderly patients and is extremely uncommon in young, otherwise healthy people. Here, we report the case of a 43-year-old woman who presented with upper gastrointestinal (GI) symptoms and iron deficiency anemia and was started on oral iron supplementation following the resolution of her acute symptoms. She soon re-presented with a severe, Helicobacter pylori -negative gastritis with iron deposition on histology. These new onset symptoms resolved rapidly with cessation of iron supplements, consistent with iron pill gastritis. In addition to the limited body of literature describing iron pill gastritis, this case serves as a reminder that any patient receiving oral iron supplementation is at a potential risk for gastritis, particularly in the setting of an ongoing GI pathology. Hence, it is important to provide continued follow-up for patients receiving iron supplementation regardless of age or comorbidity, particularly in the weeks following the start of the treatment.
Introduction
Oral iron supplements are widely used in the treatment of patients with iron deficiency anemia ( 1 ). Iron supplements are generally well-tolerated, with many patients experiencing only minor side effects including nausea/vomiting, metallic taste, staining of the teeth, or gastrointestinal (GI) distress ( 2 ). However, in rare instances, oral iron supplementation can lead to more severe symptoms, namely, acute gastritis. This is commonly referred to as “iron pill gastritis” and is diagnosed in a patient receiving iron supplementation who develops erosive gastritis with iron deposition on histopathology ( 3 , 4 ). Although iron-induced mucosal injury is rare in all patients, it is most common in the elderly ( 5 ), with only a few reported instances in young patients. Here, we report the case of a 43-year-old woman with iron deficiency anemia and unmanaged gastroesophageal reflux disease (GERD) who presented with melena and coffee-ground emesis. After her acute symptoms were resolved, she was administered oral iron supplementation with plans to follow-up in the outpatient setting. However, she rapidly developed upper GI bleeding and a severe, Helicobacter pylori ( H. pylori )-negative gastritis with iron deposits on histology. This case serves as an important reminder that, though rare, any patient receiving oral iron supplementation is at a risk for gastritis. Accordingly, it is essential to provide continued follow-up for patients receiving iron supplementation independent of age or comorbidity.
Case presentation
A 43-year-old woman presented to the emergency department complaining of intermittent melena, and coffee-ground emesis for the past two weeks. She also reported an unintentional 25-pound weight loss in recent months, which she attributed to nausea and decreased appetite. She had been diagnosed with severe GERD approximately 18 months earlier, though she was not taking any medication. Physical examination was unremarkable, and negative for abdominal pain or peritoneal signs. Lab work was significant only for normocytic anemia with hemoglobin of 10.1 g/dl (normal range 12–15.5 g/dl) with a mean corpuscular volume of 97 fl (normal range 80–100 fl). Fetal occult blood testing was negative, LDH was within normal limits, and blood urea nitrogen to creatinine ratio was unremarkable at 14:1. Additional workup showed a low serum iron of 31 mcg/dL (normal range 60–170 mcg/dL) with low transferrin (123 mg/L, normal range 11 to 307 mg/L), low total iron binding capacity (172 mcg/dL, normal range 240–450 mcg/dL), low transferrin saturation (18%, normal range 20–50%), and a ferritin of 87 ng/mL (normal range 5–116 ng/mL). After her acute symptoms had resolved, the patient was started on oral ferrous sulfate of 325 mg BID and scheduled for a non-emergent endoscopy given the likelihood of a potential GI bleed.
After five days, the patient reported bright red blood in her stool. On re-evaluation, her hemoglobin was now 7.5 g/dl, and physical examination was significant for upper quadrant and left flank tenderness. Given concern for a new or worsening upper GI bleed, an urgent esophagogastroduodenoscopy (EGD) was performed which revealed atrophic gastritis with nodular and thickened mucosa, and multiple non-bleeding ulcerations in the gastric body, antrum, and prepyloric regions ( Figure 1 ). A hemostatic clip was placed in the second portion of the duodenum, and ulcerations were biopsied, showing inflammation with pits of brown pigment consistent with iron gastropathy ( Figure 2 ). Biopsies showed no evidence of intestinal metaplasia or lymphoma, stained negative for H. pylori , and positive for iron crystals via Prussian blue staining ( Figure 2 ). Based on these observations and recent criteria regarding histologic subtypes for drug-induced GI lesions ( 6 ), the patient was diagnosed with an ischemic-type iron pill gastritis and oral ferrous sulfate was discontinued immediately. The patient was treated conservatively with omeprazole (40 mg PO daily) for her GERD, and experienced the resolution of her symptoms over the next two days, reporting no further nausea, vomiting, hematemesis, melena, or hematochezia.
Figure 1. Esophagogastroduodenoscopy showing atrophic gastritis with nodular mucosa and multiple non-bleeding ulcerations.
Figure 2. Gastric biopsy confirming the diagnosis of iron pill gastritis. Gastric lesions were biopsied and stained with H&E, showing inflammation with pits of brown pigment (yellow arrows) consistent with iron gastropathy. Tissues were also stained via Prussian blue, confirming the presence of iron crystals (yellow arrows) in the gastric mucosa.
Iron-induced mucosal injuries to the upper GI tract are well-documented. These events involve GI necrosis and strictures following an iron overdose and generally occur due to comorbidities that cause the excessive accumulation of iron including hemochromatosis, cirrhosis, and multiple blood transfusions ( 4 , 5 ). However, for patients receiving standard dose iron supplements without systemic iron overload, the etiology of mucosal injury is poorly understood.
To this end, a seminal study explored the effects of standard-dose oral iron therapy on the upper GI tract using 27 healthy volunteers. In this study, 14 participants underwent baseline endoscopy with a biopsy of the stomach and duodenum and provided a stool sample. They were then administered standard dose oral iron supplementation for two weeks, followed by a repeat endoscopy/biopsy and stool sample collection. Thirteen additional participants provided a pre-treatment stool sample, provided a pretreatment stool sample and were administered standard dose oral iron supplementation for one week, and a posttreatment stool sample was collected. All participants developed dark stools posttreatment, and nausea and diarrhea were ubiquitous. One patient had a potentially trace-positive hemoccult test. However, the authors noted that several participants showed changes in posttreatment endoscopy of the stomach, mostly in the form of erythema, small areas of subepithelial hemorrhage, and (in two patients) gastric erosions ( 7 ).
A subsequent study of 1,300 upper GI tract biopsies from 33 patients identified crystalline iron deposition in 12/1,300 biopsies, and mucosal damage in 9/1,300. The authors noted that the areas of mucosal ulceration had a significant overlap with those of iron deposition, particularly for patients with associated upper GI disorders. The authors therefore concluded that therapeutic iron supplementation can both induce and exacerbate erosive mucosal injury ( 8 ). Despite these and other studies, it remains unclear how iron pill gastritis occurs or why certain patients are affected. Recent evidence suggests that this involves iron oxidation from ferrous to ferric form, leading to epithelial injury of the esophagus and stomach ( 9 ), which can lead to mucosal injury similar to that caused by chemical burns ( 10 ). However, this area warrants continued exploration, particularly as the field reaches a consensus that iron pill gastritis is likely underdiagnosed ( 3 , 4 ).
Importantly, as our patient presented with acute GI symptoms, our case raises the question as to whether oral iron supplementation led to a new ulcerative gastritis, or exacerbated an existing, more insidious gastritis. In addition, it also raises questions as to whether the standard approach to iron supplementation is appropriate for all patients. For years, the treatment guidelines for iron deficiency anemia in adults have recommended a daily intake of 100–200 mg of oral iron, administered as either one or two doses. However, emerging evidence suggests that less frequent dosing may be more effective than daily dosing. For example, two recent open-label trials measured iron absorption in iron-deficient, non-anemic adult women receiving various dosing regimens of oral iron. The authors found that total iron absorption was superior in women receiving every-other-day iron dosing when compared to those receiving daily dosing. Additionally, there was no significant difference in iron absorption between women receiving a single daily dose vs. twice-daily divided doses ( 11 ).
A subsequent study in 19 women with iron deficiency anemia also determined that every-other-day iron dosing improved overall iron absorption when compared to once-daily dosing. The authors also reported a 40% decrease in Grade I and II GI side effects (abdominal pain, nausea, vomiting, and diarrhea), although this difference was not statistically significant likely due to the small sample size ( 12 ). Hence, this too warrants continued investigation, particularly regarding treatment-related adverse events in vulnerable patient populations.
Finally, there are also emerging data suggesting that intravenous (IV) iron sucrose may have a better safety profile and efficacy than oral iron supplements ( 13 ). For example, oral iron supplements are associated with higher GI side effects than either IV iron or placebo ( 14 , 15 ). Classically, this approach has been reserved for patients with chronic iron deficiency anemia and comorbid chronic kidney disease, irritable bowel disease, or those who develop intolerable GI side effects from oral iron supplementation ( 16 – 18 ). However, given the favorable toxicity profile, IV administration may provide a safer and reasonable alternative to oral iron supplementation in complex patients like ours. While this requires further study, this case serves as an important reminder that careful follow-up is required for all patients started on iron supplementation, particularly those with ongoing upper GI pathologies.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RK and DP drafted the manuscript. ST edited the manuscript. All authors read and approved the final version of the manuscript.
This work was supported by NIH F30CA236031 to DP.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. (2007) 370:511–20. doi: 10.1016/S0140-6736(07)61235-5
CrossRef Full Text | Google Scholar
2. Nguyen M, Tadi P. Iron Supplementation. Treasure Island, FL: StatPearls (2022).
Google Scholar
3. Onorati M, Nicola M, Renda A, Lancia M, Di Nuovo F. Iron overload in gastric mucosa: underdiagnosed condition rarely documented in clinical and pathology reports. Cureus. (2020) 12:e8234. doi: 10.7759/cureus.8234
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Sunkara T, Caughey ME, Nigar S, Olivo R, Gaduputi V. Iron pill gastritis: an under diagnosed condition with potentially serious outcomes. Gastroenterol Res. (2017) 10:138–40. doi: 10.14740/gr804w
5. Haig A, Driman DK. Iron-induced mucosal injury to the upper gastrointestinal tract. Histopathology. (2006) 48:808–12. doi: 10.1111/j.1365-2559.2006.02448.x
6. De Petris G, Caldero SG, Chen L, Xiao SY, Dhungel BM, Spizcka AJ, et al. Histopathological changes in the gastrointestinal tract due to medications: an update for the surgical pathologist (part II of II). Int J Surg Pathol. (2014) 22:202–11.
7. Laine LA, Bentley E, Chandrasoma P. Effect of oral iron therapy on the upper gastrointestinal tract. A prospective evaluation. Dig Dis Sci. (1988) 33:172–7. doi: 10.1007/BF01535729
8. Abraham SC, Yardley JH, Wu TT. Erosive injury to the upper gastrointestinal tract in patients receiving iron medication: an underrecognized entity. Am J Surg Pathol. (1999) 23:1241–7. doi: 10.1097/00000478-199910000-00009
9. Aruoma OI, Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. (1987) 241:273–8. doi: 10.1042/bj2410273
10. Hashash JG, Proksell S, Kuan SF, Behari J. Iron pill-induced gastritis. ACG Case Rep J. (2013) 1:13–5. doi: 10.14309/crj.2013.7
11. Stoffel NU, Cercamondi CI, Brittenham G, Zeder C, Geurts-Moespot AJ, Swinkels DW, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. (2017) 4:e524–33. doi: 10.1016/S2352-3026(17)30182-5
12. Stoffel NU, Zeder C, Brittenham GM, Moretti D, Zimmermann MB. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. (2020) 105:1232–9. doi: 10.3324/haematol.2019.220830
13. Das SN, Devi A, Mohanta BB, Choudhury A, Swain A, Thatoi PK. Oral versus intravenous iron therapy in iron deficiency anemia: an observational study. J Fam Med Prim Care. (2020) 9:3619–22. doi: 10.4103/jfmpc.jfmpc_559_20
14. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, Haya-Palazuelos J, Ciria-Recasens M, Manasanch J, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. (2013) 29:291–303. doi: 10.1185/03007995.2012.761599
15. Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. (2015) 10:e0117383. doi: 10.1371/journal.pone.0117383
16. Cooke M, Lamplugh A, Naudeer S, Edey M, Bhandari S. Efficacy and tolerability of accelerated-dose low-molecular-weight iron dextran (cosmofer) in patients with chronic kidney disease. Am J Nephrol. (2012) 35:69–74. doi: 10.1159/000334877
17. Macdougall IC. Strategies for iron supplementation: oral versus intravenous. Kidney Int Suppl. (1999) 69:S61–6. doi: 10.1046/j.1523-1755.1999.055Suppl.69061.x
18. Auerbach M, Ballard H, Glaspy J. Clinical update: intravenous iron for anaemia. Lancet. (2007) 369:1502–4. doi: 10.1016/S0140-6736(07)60689-8
Keywords : gastritis, oral iron supplementation, gastroesophageal reflux disease, emergency medicine, medication adverse effect, gastrointestinal tract
Citation: Koch RM, Tchernodrinski S and Principe DR (2022) Case report: Rapid onset, ischemic-type gastritis after initiating oral iron supplementation. Front. Med. 9:1010897. doi: 10.3389/fmed.2022.1010897
Received: 03 August 2022; Accepted: 05 October 2022; Published: 03 November 2022.
Reviewed by:
Copyright © 2022 Koch, Tchernodrinski and Principe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel R. Principe, cHJpbmNpcGVAdWljLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
Dr. John A. Weems (Medicine): A 29-year-old woman was evaluated at a primary care clinic affiliated with this hospital because of nausea, vomiting, and diarrhea.
case study gastritis - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. A 68-year-old Hispanic female presented with nausea, vomiting, abdominal pain, and fever for several weeks.
In this report, we present an 18-year-old man without any past medical history who was evaluated after an episode of syncope. Evaluation revealed a case of chronic H. pylori gastritis leading to gastrointestinal (GI) bleeding and weight loss, and his syncope was the byproduct of symptomatic anemia and physical exertion. Pediatricians should ...
Acquire adequate knowledge on Acute Gastritis with moderate Dehydration. Understand the pathophysiology of the case being presented. Understand the role of drug therapy in managing the client’s diagnosis. interactions. Encourage interaction to the family of the patient to establish rapport.
This document is a case study on acute gastritis submitted by a nursing student. It includes an introduction describing acute gastritis and its prevalence. The objectives are to demonstrate knowledge of acute gastritis and provide appropriate nursing care.
Acute phlegmonous gastritis (APG) is an extremely uncommon and potentially rapid fatal systemic infection with very few reported cases in the literature. This case report demonstrates a case of idiopathic APG in an afebrile, otherwise healthy ...
Emphysematous gastritis (EG) is a rare condition characterized by air within the gastric wall with signs of systemic toxicity. The optimal management for this condition and the role of surgery is still unclear. We here report three cases of EG successfully managed non-operatively.
The document provides an overview of a case study about a 28-year-old male patient diagnosed with acute gastritis. The introduction defines gastritis and describes common causes, symptoms, and diagnostic tests.
Relying on essential information about the etiology and staging of a case of gastritis, clinicians can safely proceed with the most appropriate therapy, and decide whether and what type of follow-up is required [49, 50].
Here, we report the case of a 43-year-old woman who presented with upper gastrointestinal (GI) symptoms and iron deficiency anemia and was started on oral iron supplementation following the resolution of her acute symptoms. She soon re-presented with a severe, Helicobacter pylori-negative gastritis with iron deposition on histology. These new ...