An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Active placebo versus standard placebo control interventions in pharmacological randomised trials
David rt laursen, camilla hansen, asger sand paludan-müller, asbjørn hróbjartsson.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Collection date 2020.
This is a protocol for a Cochrane Review (methodology). The objectives are as follows:
To estimate differences in effects between pharmacological active placebo interventions and standard placebo interventions in randomised trials.
Placebo control interventions are used in randomised clinical trials to blind participants and healthcare providers, making them unaware of the allocated treatment. A trial in which these people are aware of a participant's treatment allocation may potentially be biased. For example, patients may report what they think will please the doctors (i.e. response bias) ( Hróbjartsson 2011a ), or experience or interpret symptoms differently (i.e. placebo effect) ( Hróbjartsson 2002 ), or outcome assessors may rate one of the interventions more favourably (i.e. observer bias) ( Schulz 2002 ; Hróbjartsson 2011a ). The use of a placebo control intervention to induce and maintain blinding reduces the risk of bias ( Schulz 2002 ; Hróbjartsson 2011a ).
Placebos enable blinding by mimicking the experience of receiving an experimental intervention without containing any supposed therapeutic components. Placebos are often associated with pharmacological trials where they are typically designed to be indistinguishable in appearance, smell, taste and texture from the drug under investigation.
However, some experimental drug interventions may have instantaneous and perceptible psychotropic or adverse effects with a risk of patient unblinding. Examples of such drugs and their adverse effects are. selective serotonin reuptake inhibitors inducing nausea, insomnia and nervousness ( Greenberg 1994 ); tricyclic antidepressants inducing anticholinergic effects, such as mouth dryness ( Moncrieff 2004 ); methylphenidate inducing euphoria and nausea ( Storebø 2015 ); and lithium inducing tremor ( Marini 1976 ). Inadequate matching between active drugs and standard placebo controls by external characteristics is not uncommon and poses a potential risk for unblinding ( Bello 2016 ). Similarly, this risk of unblinding is also likely to be an issue when placebo controls do not match internal characteristics such as psychotropic or adverse effects.
To address the potential issue of unblinding due to adverse effects, some drug trials have used a so‐called active placebo instead of standard placebo as their control intervention ( Jensen 2017 ).
Description of the methods being investigated
Pharmacological active placebos are designed to imitate the external characteristics and also the internal sensations of receiving the treatment, by mimicking some of its psychotropic or adverse effects. One example is atropine, which can imitate the anticholinergic effects of tricyclic antidepressants ( Thomson 1982 ).
Some non‐pharmacological placebos can also be regarded as active placebos, closely mimicking the experience of receiving active treatment. For example, a device intervention such as transcutaneous electrical nerve stimulation (TENS) may be compared to a sham TENS, which will provide subtherapeutic levels of stimulation just above the minimal sensory threshold ( Tucker 2015 ). Surgical placebos can be considered a more invasive form of active placebo with adverse reactions from the surgery itself and have their own ethical challenges ( Wartolowska 2014 ). Psychological placebos, on the other hand, are different from the other types of nonpharmacological placebo in that they do not match the intervention ( Hróbjartsson 2011b ). For our Cochrane Review, we will only consider pharmacological active placebos.
How these methods might work
Active placebos aim to more closely simulate the experience of receiving the experimental intervention. This reduces the perceptible differences between the experimental and control treatments and will ideally reduce the risk of patients and healthcare providers identifying the allocated treatment. Active placebos may thereby reduce the risk of bias due to unblinding and prevent overestimation of treatment benefits ( Jensen 2017 ).
Thus, active placebos can, in theory, more effectively control for placebo effects and certain biases in trials. However, it is not entirely clear what could cause the effects of active placebos to differ from those of standard placebos. One possible explanation may concern expectations. For example, physical placebos are associated with higher effects than standard pharmacological placebos ( Hróbjartsson 2010 ). One could therefore hypothesise that the more extensive or theatrical an intervention is, be it active placebo or surgical placebo, the higher are the expectations of receiving an effective treatment, which in turn could lead to a greater treatment response ( Meissner 2011 ; Rief 2011 ). Hence, active placebos may increase the expectation of receiving the actual treatment through experiencing of adverse effects, thus increasing the placebo effect.
On the other hand, active placebos also carry a risk of having unintended therapeutic effects on the outcome of interest, thereby potentially underestimating the true effect of the intervention ( Jensen 2017 ).
Why it is important to do this review
The history of active placebos goes back to the 1950s and the early development of the randomised trial ( Lasagna 1955 ; Lasagna 1958 ). In recent methodology literature, this part of trial design has received little attention ( Salamone 2000 ; Edward 2005 ; Wartolowska 2014 ), with few studies focusing on use and impact of active placebos ( Moncrieff 2003 ; Moncrieff 2004 ; Jensen 2017 ). A recent review by Jensen et al. aimed to record all drug trials using active placebos, including trials comparing experimental drug to active placebo ( Jensen 2017 ). They noted that active placebos were rarely and inconsistently used. For example, active placebos were used in trials of selective serotonin reuptake inhibitors for pain, but not for depression.
The use of active placebos, therefore, does not seem always to be based on rational and transparent choices. This highlights a likely need to develop guidelines for use of active placebos in trials, and to answer the central empirical question: do active placebos, on average, reduce the risk of bias due to unblinding; and if so, under what circumstances?
Criteria for considering studies for this review
Types of studies.
We will include all randomised clinical and preclinical trials that allocate participants to standard placebo intervention and to active placebo intervention.
We define ‘randomised’ to mean any trial denoted explicitly as such, or reporting a design which can be assumed to have a random allocation schedule, e.g. Latin square design.
We define a ‘clinical trial’ to mean any trial investigating the effects of a healthcare intervention on patients or on participants at risk of disease. We define a ‘preclinical trial’ to mean any trial investigating the effects of a healthcare intervention on healthy participants. By ‘healthcare intervention’, we mean that the intervention is or could potentially be used for the treatment or prevention of disease.
We will include trials with a parallel or crossover design, but not split‐body or cluster randomisation.
Types of data
The data for this review will consist of data from trial publications, including trial characteristics, information on the standard and active placebo used and outcome results such as patient‐reported continuous outcomes and observer‐reported continuous outcomes.
Types of methods
We will include trials with a randomised head‐to‐head comparison of pharmacological active placebo versus standard placebo. We will not consider trials having only comparisons of experimental drug versus active placebo (which have been recorded in Jensen 2017 ) or experimental drug versus standard placebo.
We define ‘active placebo’ as any intervention labelled as such, or any intervention stated to imitate the instantaneous and perceptible psychotropic or adverse effects of the experimental intervention (e.g. anticholinergic effects of tricyclic antidepressants, or tremor from lithium), but without any known or suspected benefit on the outcomes under investigation. The active placebo may also be labelled as, for example, ‘active control’ or ‘active control treatment’. We will only consider trials where the active placebo is designated a priori as such. We will exclude trials reinterpreting an intervention designed to be experimental as an active placebo, for example when the trial authors reflect on their results in the discussion section of their trial report.
We define ‘standard placebo’ as any intervention labelled as such, or any intervention designed to only mimic the external sensory properties of the experimental intervention, such as appearance, smell, taste and texture, but not designed to imitate its psychotropic or adverse effects. The standard placebo may also be labelled as, for example, ‘inert placebo’, ‘inactive placebo’, ‘true placebo’ or ‘sham’.
We will include trials both with and without a study group treated with an experimental drug, i.e. both three (or more) and two‐group trials.
Types of outcome measures
We will not restrict the study selection based on outcome measures.
The outcomes of interest for our review are listed below.
Primary outcomes
The standardised mean difference (SMD) for patient‐reported outcomes of benefit (e.g. visual analogue scale (VAS) for pain, symptom score)
at the earliest post‐treatment time point
Secondary outcomes
SMD for patient‐reported outcomes of benefit
at the latest follow‐up time point
SMD for blinded observer‐reported outcomes of benefit (e.g. rating scale)
at the earliest post‐treatment time point and
SMD for any type of outcomes of harm (e.g. adverse reaction assessment)
Odds ratio (OR) for any type of outcomes of harm (e.g. adverse reactions)
Difference in attrition rates
Difference in co‐intervention rates
Difference in mean co‐intervention use
Search methods for identification of studies
We will use the following sources to screen for eligible trial publications:
the 89 randomised trials included in Jensen 2017 , which is a systematic review aimed at identifying trials with pharmacological active placebo groups that had been reported before 15 January 2015
update of the database search done for Jensen 2017 from January 2015 onwards
more specific database searches and searching other sources
We expect that relevant studies may be difficult to identify in standard database searches, due to differing and non‐standardised ways of reporting active placebos. Our search will mainly focus on points 1 and 3 above, because most relevant studies are likely to have been found by Jensen 2017 or be found by searching sources other than the standard databases.
Updated database search
For the updated electronic search from Jensen 2017 , we will search PubMed and Cochrane Central Register of Controlled Trials (CENTRAL) for potentially relevant trial publications added in or since 2015. We will use the search strategy developed by Jensen 2017 (see Appendix 1 ).
Specific database search and searching other resources
We will perform a more specific search in Google Scholar and Ovid Embase (including Embase Classic) aimed at finding randomised trials comparing active and standard placebos, with no time restrictions (see Appendix 1 ).
We will screen reference lists of relevant publications for potentially eligible studies and use Web of Science to search for potentially eligible citations to the relevant publications ( Horsley 2011 ).
We will perform searches in trial registries (e.g. ClinicalTrials.gov, WHO ICTRP) and other databases (e.g. ProQuest) and contact relevant experts and authors for other potentially relevant trial publications.
Data collection and analysis
Selection of studies.
For the above sources, one review author (DL) will screen titles and abstracts and perform obvious exclusions. For Google Scholar, one review author (DL) will screen text excerpts and, if needed, the full text of the first 100 hits of each search term and perform obvious exclusions.
Then, two review authors (DL and a second review author) will screen relevant publications in full for eligibility. We will resolve any disagreements by discussion.
Data extraction and management
Two review authors (DL and a second review author) will extract data from eligible studies. The data are classified as: basic data, outcomes, covariates. We will resolve any disagreements by discussion.
For basic data, we plan to extract publication and first author information, trial characteristics (clinical or preclinical, clinical area, country, trial design, information on funding and conflicts of interest, type of participants or condition, interventions and comparators), information on trial conduct (duration of treatment and follow‐up, description of randomisation, blinding and co‐interventions), as well as participant flow numbers (number of participants screened, enrolled and randomised) (see Appendix 2 for more details).
For all outcome results reported, we plan to extract the following data: basic outcome information (e.g. outcome type: continuous; domain and instrument: pain on VAS scale; directionality: lower is better), time for registration (e.g. two weeks post‐treatment).
For continuous outcomes, we plan to extract the summary data from the active placebo and standard placebo groups which are needed for a calculation of the standardised mean difference (SMD): the mean, standard deviation (SD) (or another measure of variability, e.g. standard error (SE) or confidence interval (CI)) and number of patients (N). For dichotomous outcomes, we plan to extract the summary data from each group needed for a calculation of the OR: number of patients registered as having the event and total number of patients assessed.
If summary data are not available, but only effect estimates (e.g. mean difference, SMD, OR), we will extract these instead along with measures of variability (e.g. CI, P value).
For each outcome, we will prefer the most complete analysis based on available patients as they are randomised. If multiple publications report on the same trial, we will use data from all the sources. In case of discrepancies, we will attempt to contact the study authors.
Selection of review outcomes in individual trials
Two review authors will independently select appropriate primary and secondary outcomes from each trial and will resolve any disagreement by discussion. The preferred order is:
continuous outcomes, otherwise dichotomous outcomes (for patient‐reported and observer‐reported outcomes)
primary outcomes, if noted, otherwise the clinically most relevant outcomes
absolute values, otherwise change scores
for patient‐reported outcomes:
symptom‐specific outcomes (e.g. pain), otherwise global outcomes (e.g. quality of life score)
private (e.g. pain), otherwise potentially observable (e.g. emesis)
for observer‐reported outcomes:
subjective interactive (e.g. rating scales with patient contact, health‐care provider decision outcomes), otherwise subjective pure observational, otherwise objective outcomes (e.g. non‐repeatable measurements such as blood samples)
for outcomes of harm:
patient‐reported outcomes, otherwise observer‐reported outcomes
then same principles as above, except we select both a continuous and a dichotomous outcome of harm
For the active placebo in each trial, we will grade the adequacy of the active placebo on a ranking scale from 1 to 5, where we will consider the quality, intensity and rapidness of the psychotropic effects and the similarity to the experimental treatment, as a measure of the likelihood to cause unblinding compared to standard placebo. We will base grading primarily on information from the trial publication, but also using relevant references and an informal probing of the literature. Two review authors will assess the adequacy and disagreements will be resolved by discussion.
Based on the same principles, we will also grade the risk of unintended therapeutic effects from the active placebo on a ranking scale from 1 to 5.
Assessment of risk of bias in included studies
We will assess the risk of bias in the included trials using the following domains from the 2011 'Risk of bias' tool ( Higgins 2011 ): selection bias, attrition bias, reporting bias and other bias. We will not assess those related to blinding (performance bias and detection bias) since these domains are the subjects of the investigation in this review.
Unit of analysis issues
For crossover trials, we will attempt to use outcome results from all crossover periods using reported or estimated correlation coefficients. Alternatively, we will use data from the first period before crossover, if available.
Dealing with missing data
If data are partly or completely missing and not available for our analyses, we will attempt to contact study authors ( Young 2011 ) or will impute the missing values using estimates from other studies (e.g. same scales in similar trials).
Data synthesis
Data conversion.
For continuous outcomes, if the necessary summary data for each intervention group are available (mean, SD, and number of participants), we will calculate an estimate of the SMD and its standard error directly based on these numbers. If the above summary data are not reported directly, we will attempt to estimate them using other measures, e.g. standard error, CIs, test statistics, P values.
For dichotomous outcomes, if the necessary summary data for each intervention group are available, we will calculate the OR based on a standard 2x2 table. If the dichotomous outcome has been selected for our review outcomes because of the unavailability of continuous outcomes, we will convert the OR to a SMD ( Hasselblad 1995 ; Chinn 2000 ).
If summary data for each intervention group are not available for the given outcome of interest, but rather an estimate is available (e.g. mean difference or SMD, along with a measure of variability), we will use these in our data analysis.
We will convert directions of outcomes, such that SMD < 0 and OR < 1 indicate greater benefit of active placebo compared to standard placebo.
Data analysis
We will use the inverse‐variance method to perform random‐effects meta‐analyses for primary and secondary outcomes. We will stratify for type of trial, presenting clinical and preclinical trials separately. To explore heterogeneity, we will calculate I 2 , τ 2 and prediction intervals.
For continuous primary and secondary outcomes, we will use the SMD and corresponding standard error from each trial, calculated or estimated as described above. If this is not possible due to missing data, the trial will be omitted from the given meta‐analysis and the results from such trials will be summarized qualitatively. Two review authors (DL and a second review author) will decide whether or not a trial will be included in the meta‐analysis.
For dichotomous secondary outcomes (e.g. attrition, co‐interventions), we will perform a meta‐analysis using OR.
Summarising the evidence
Based on the main and additional analyses, we will summarise the evidence qualitatively to answer the review's objective. We will reflect on the quality of evidence informally based on the same components as in the GRADE assessment, e.g. risk of bias, inconsistency, publication bias ( Guyatt 2011 ).
Subgroup analysis and investigation of heterogeneity
To explore potential heterogeneity, we plan to perform the following metaregression analysis for our primary and secondary outcomes with the two covariates noted previously as variables:
adequacy of the active placebo, on a ranking scale from 1 to 5
risk of unintended therapeutic effects, on a ranking scale from 1 to 5
Sensitivity analysis
To study the robustness of our results, we plan to perform the following sensitivity analyses for our primary and secondary outcomes:
continuous outcome data only, excluding dichotomous outcome data that have been converted to SMD
inclusion of excluded, but nearly eligible trials
Protocol first published: Issue 7, 2020
Acknowledgements
We thank Lasse Østengaard, Research Librarian at the University Library of Southern Denmark and PhD student at the Centre for Evidence‐Based Medicine Odense (CEBMO), for valuable help in developing the search strategies. We thank Karsten Juhl Jørgensen, Acting Director of the Nordic Cochrane Centre, for valuable comments to the protocol.
Appendix 1. Search strategy
Screening trials included in jensen 2017 study.
After correspondence to Jensen 2017 we retrieved the list of 89 trials using an active placebo.
Updated systematic search
We will base the updated search on the strategy from Jensen 2017 , performed in PubMed and Cochrane Central Register of Controlled Trials (CENTRAL) from 2015 onwards:
“active placebo” OR “active placebos” OR “active control treatment” OR “active control” OR “sham diphenhydramine” OR “diphenhydramine placebo” OR “sham atropine” OR “atropine placebo” OR “sham benztropine” OR “sham benzodiazepine” OR “histamine placebo” OR “benztropine placebo” OR “benzodiazepine placebo”
Focused electronic search
Our search strategy is in part informed by Jensen 2017 . We have the following draft for a search strategy for Ovid EMBASE:
For full‐text search in Google Scholar, we plan to perform a search on the search strings (#1 and #3), #5, #8 and #9 separately, and without the subject headings. We plan to screen the first 100 search results for each block.
Appendix 2. Data extraction
Publication information
Corresponding author
Contact information
Country of first author
Trial characteristics
Study IDs and other identifiers
Clinical or pre‐clinical trial
Clinical area (e.g. by journal topic or author’s affiliated department)
Country or countries of recruitment
Trial design, e.g. parallel design
Information on funding and conflicts of interest (e.g. non‐industry‐funded, and reports no conflicts of interest)
Type of participants and their conditions
Trial setting
Types of interventions and comparators
Description of active and standard placebo (e.g. design and mode of action)
Trial conduct
Duration of treatment and duration of follow‐up
Description of randomisation procedures
Including random allocation or quasi‐random allocation
Description of blinding procedures
Including blinding status on key trials persons, tests of blinding success and reports of unblinding
Description of co‐interventions
Trial results other than for specific outcome measures
Participant flow
Including number of participants randomised to each study intervention, attrition for each study arm and reasons for attrition
Contributions of authors
AH conceived the idea for the systematic review. DL and AH primarily wrote the protocol, and CH and AP contributed. All authors read and approved the final protocol version.
Sources of support
Internal sources.
University of Southern Denmark (SDU), Denmark
Scholarship from the SDU, which funds one year of DL’s PhD employment
Nordic Cochrane Centre, Denmark
Funds 1.5 year of DL’s PhD employment
External sources
No sources of support supplied
Declarations of interest
DL has no known financial or non‐financial conflicts of interest. CH has no known financial or non‐financial conflicts of interest. AP has no known financial or non‐financial conflicts of interest. AH has no known financial or non‐financial conflicts of interest.
Additional references
- Bello S, Wei M, Hilden J, Hróbjartsson A. The matching quality of experimental and control interventions in blinded pharmacological randomised clinical trials: a methodological systematic review. BMC Medical Research Methodology 2016;16:18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Statistics in Medicine 2000;19(22):3127-31. [ DOI ] [ PubMed ] [ Google Scholar ]
Edward 2005
- Edward SJL, Stevens AJ, Braunholtz DA, Lilford RJ, Swift T. The ethics of placebo-controlled trials: a comparison of inert and active placebo controls. World Journal of Surgery 2005;29(5):610-4. [ DOI ] [ PubMed ] [ Google Scholar ]
Greenberg 1994
- Greenberg RP, Bornstein RF, Zborowski MJ, Fisher S, Greenberg MD. A meta-analysis of fluoxetine outcome in the treatment of depression. Journal of Nervous and Mental Disease 1994;182(10):547-51. [ DOI ] [ PubMed ] [ Google Scholar ]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [ DOI ] [ PubMed ] [ Google Scholar ]
Hasselblad 1995
- Hasselblad V, Hedges LV. Meta-analysis of screening and diagnostic tests. Psychological Bulletin 1995;117(1):167-78. [ DOI ] [ PubMed ] [ Google Scholar ]
Higgins 2011
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Horsley 2011
- Horsley T, Dingwall O, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database of Systematic Reviews 2011, Issue 8. Art. No: MR000026. [DOI: 10.1002/14651858.MR000026.pub2] [PMID: ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Hróbjartsson 2002
- Hróbjartsson A. What are the main methodological problems in the estimation of placebo effects? Journal of Clinical Epidemiology 2002;55(5):430-5. [ DOI ] [ PubMed ] [ Google Scholar ]
Hróbjartsson 2010
- Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD003974. [DOI: 10.1002/14651858.CD003974.pub3] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Hróbjartsson 2011a
- Hróbjartsson A, Boutron I. Blinding in randomized clinical trials: imposed impartiality. Clinical Pharmacology and Therapeutics 2011;90(5):732-6. [ DOI ] [ PubMed ] [ Google Scholar ]
Hróbjartsson 2011b
- Hróbjartsson A, Miller FG. Placebo in nonpharmacological randomized trials. In: Boutron I, Ravaud P, Moher D, editors(s). Randomized Clinical Trials of Nonpharmacological Treatments. Boca Raton, Florida: Chapman and Hall/CRC, 2011:11-26. [ Google Scholar ]
Jensen 2017
- Jensen JS, Bielefeldt AØ, Hróbjartsson A. Active placebo control groups of pharmacological interventions were rarely used but merited serious consideration: a methodological overview. Journal of Clinical Epidemiology 2017;87:35-46. [ DOI ] [ PubMed ] [ Google Scholar ]
Lasagna 1955
- Lasagna L. The controlled clinical trial: theory and practice. Journal of Chronic Diseases 1955;1(4):353-67. [ DOI ] [ PubMed ] [ Google Scholar ]
Lasagna 1958
- Lasagna L, Meier P. Clinical evaluation of drugs. Annual Review of Medicine 1958;9:347-54. [ DOI ] [ PubMed ] [ Google Scholar ]
Marini 1976
- Marini JL, Sheard MH, Bridges CI, Wagner E. An evaluation of the double-blind design in a study comparing lithium carbonate with placebo. Acta Psychiatrica Scandinavica 1976;53(5):343-54. [ DOI ] [ PubMed ] [ Google Scholar ]
Meissner 2011
- Meissner K, Kohls N, Colloca L. Introduction to placebo effects in medicine: mechanisms and clinical implications. Philosophical Transactions of the Royal Society B: Biological Sciences 2011;366(1572):1783-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Moncrieff 2003
- Moncrieff J. A comparison of antidepressant trials using active and inert placebos. International Journal of Methods in Psychiatric Research 2003;12(3):117-27. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Moncrieff 2004
- Moncrieff J, Wessely S, Hardy R. Active placebos versus antidepressants for depression. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD003012. [DOI: 10.1002/14651858.CD003012.pub2] [PMID: ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rief W, Bingel U, Schedlowski M, Enck P. Mechanisms involved in placebo and nocebo responses and implications for drug trials. Clinical Pharmacology & Therapeutics 2011;90(5):722-6. [ DOI ] [ PubMed ] [ Google Scholar ]
Salamone 2000
- Salamone JD. A critique of recent studies on placebo effects of antidepressants: importance of research on active placebos. Psychopharmacology 2000;152(1):1-6. [ DOI ] [ PubMed ] [ Google Scholar ]

Schulz 2002
- Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002;359(9307):696-700. [ DOI ] [ PubMed ] [ Google Scholar ]
Storebø 2015
- Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD009885. [DOI: 10.1002/14651858.CD009885.pub2] [PMID: ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Thomson 1982
- Thomson R. Side effects and placebo amplification. British Journal of Psychiatry: the Journal of Mental Science 1982;140:64-8. [ DOI ] [ PubMed ] [ Google Scholar ]
Tucker 2015
- Tucker DL, Rockett M, Hasan M, Poplar S, Rule SA. Does transcutaneous electrical nerve stimulation (TENS) alleviate the pain experienced during bone marrow sampling in addition to standard techniques? A randomised, double-blinded, controlled trial. Journal of Clinical Pathology 2015;68(6):479-83. [ DOI ] [ PubMed ] [ Google Scholar ]
Wartolowska 2014
- Wartolowska K, Judge A, Hopewell S, Collins GS, Dean BJF, Rombach I, et al. Use of placebo controls in the evaluation of surgery: systematic review. BMJ 2014;348:g3253. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Young T, Hopewell S. Methods for obtaining unpublished data. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: MR000027. [DOI: 10.1002/14651858.MR000027.pub2] [PMID: ] [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (141.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Placebo interventions, placebo effects and clinical practice
Klaus linde, margrit fässler, karin meissner.
- Author information
- Copyright and License information
Author for correspondence ( [email protected] ).
This article reviews the role of placebo interventions and placebo effects in clinical practice. We first describe the relevance of different perspectives among scientists, physicians and patients on what is considered a placebo intervention in clinical practice. We then summarize how placebo effects have been investigated in randomized controlled trials under the questionable premise that such effects are produced by placebo interventions. We further discuss why a shift of focus from the placebo intervention to the overall therapeutic context is necessary and what research methods can be used for the clinical investigation of the relevance of context effects. In the last part of the manuscript, we discuss why placebo or context effects are seen as positive in clinical practice when they are associated with active treatments, while placebo interventions pose major ethical and professional problems and have to be avoided.
Keywords: clinical practice, placebo, placebo effects, randomized controlled trials, ethics
1. Introduction
There are three major areas in which placebo interventions have an important role: (i) as control interventions in experimental studies to determine specific effects and to reduce bias by enabling blinding; (ii) as experimental interventions in placebo research to study placebo effects; (iii) as a tool in clinical practice. If one searches a major bibliographical database such as Medline for references including the word placebo, the overwhelming majority of articles identified are either placebo-controlled trials or articles referring to such trials. A small minority of articles are review articles on general or specific aspects of the placebo phenomenon, or original reports of experimental placebo research. Only a very small number of articles report empirical investigations or are essays of placebo use or placebo effects in routine practice. In this article, we first describe the relevance of different perspectives among scientists, physicians and patients on what is considered a placebo intervention in clinical practice. We then summarize how placebo effects have been investigated in clinical research under the questionable premise that such effects are produced by placebo interventions. We further discuss why a shift of focus from the placebo intervention to the overall therapeutic is necessary and what research methods can be used for the clinical investigation of the relevance of context effects. In the last part of the manuscript, we discuss why placebo or context effects are seen as positive in clinical practice when they are associated with active treatments, while placebo interventions have to be avoided.
2. What is a placebo intervention in clinical practice?
According to the classical definition by Shapiro & Morris [ 1 , p. 371] ‘a placebo is defined as any therapy or component of therapy used for its nonspecific, psychological, or psychophysiological effect, or that is used for its presumed specific effect, but is without specific activity for the condition being treated’. Shapiro & Morris further distinguish pure placebos, which are ‘treatments that are devoid of active, specific components’, and impure placebos, which ‘contain non-placebo components’ (p. 372). While this definition has been severely criticized on a conceptual level [ 2 , 3 ], it is summarizing well the implicit view of placebo interventions in biomedicine. We will not discuss conceptual issues here, but we will demonstrate—by using simple case scenarios of interventions classifying as placebos according to this definition—that, in clinical practice, it is often quite difficult to decide what should actually be considered a placebo ( table 1 ). These difficulties are owing to the fact that the perspective of the definition by Shapiro & Morris is scientific, while physicians providing an intervention and patients receiving it might hold a different view.
Five clinical scenarios and related views of providers, patients and scientists whether the intervention provided is to be considered a placebo.
In scenario 1, a typical pure placebo (a saline injection) is administered to a pain patient. Both the provider and the scientist ‘know’ that the intervention is a placebo. The patient is informed in a deceptive manner which makes him believe he is receiving a ‘true’ treatment. If he were to be informed correctly he would also consider the treatment a placebo.
According to surveys, between 17 and 80 per cent of physicians and between 51 and 100 per cent of nurses have used pure placebos intentionally at some point in their professional career [ 4 ]. However, the data also indicate that the actual frequency is rare, because pure placebos are usually applied only once or a few times to a small minority of patients.
There are three basic motivational patterns for such intentional use of a pure placebo. First, the physician aims primarily to promote the patient's wellbeing. For example, in a young patient suffering from severe headaches at risk of becoming dependent on morphine, a physician tried to reduce this risk by substituting some applications with placebo injections without informing the patient or his parents [ 5 ]. In another example, a woman with newly diagnosed advanced cancer for which a curative treatment was not possible still had great hopes of being cured. In order not to dash the patient's hopes and making her remaining time unbearable, she received a placebo intervention described as a form of cancer treatment [ 6 ]. While in both cases the patient was informed in a deceptive manner and the physician placed the relevance of his intent to help over the patient's autonomy and the ideal of shared decision-making, some authors believe that such placebo applications can be ethically justifiable (e.g. [ 7 ]). Physicians move in a grey area, and opinions on the acceptability of using pure placebos vary. The first is a real case in which the mother of the patient filed a professional grievance against the physician and a nurse [ 5 ]. The second case is fictive from a survey asking both physicians and patients to assess the acceptability of the placebo treatment. Sixty-three per cent of participating patients and 18 per cent of physicians found the procedure acceptable as it was likely to preserve the patient's hope [ 6 ].
A second motivational pattern could be summarized as ‘convenience’ [ 8 ]. For example, several surveys have found that pure placebos are given to difficult or complaining patients, or to avoid conflicts with a patient [ 9 – 13 ]. While understandable to some extent in a busy routine practice, such actions seem highly problematic both on a professional and on an ethical level [ 8 ]. It is likely that in reality many intentional applications of pure placebos are owing to a mixture of both the aim to promote wellbeing and convenience.
A third pattern, which seems to have become more and more infrequent but still occurs, is the use of placebo for diagnostic purposes. In such cases placebos are given to see whether the complaints are ‘real’ or ‘simulated’ or ‘only psychological’ [ 4 ]. Such a use is not only ethically problematic, but also contrary to the evidence which clearly shows that ‘real’ complaints can react to placebo applications.
Scenario 2 involves a patient with suspected viral upper respiratory tract infection who asks to receive the antibiotic that has helped so greatly in previous infections, and the physician complies. Antibiotics are potent and highly effective drugs when applied adequately but they are not indicated in viral infections. Therefore, this is considered as a classical example of an impure placebo. Obviously, the patient considers the treatment specific. The physician considers the antibiotic non-indicated, but there might be some uncertainty regarding the viral origin or a risk of bacterial super-infection. Based on general pathophysiological reasoning and clinical trial data, the scientist makes a general judgement that antibiotics do not have an effect over placebo in patients with viral infection.
Surveys show that the non-indicated use of active drugs is much more frequent than the use of pure placebos [ 11 , 13 , 14 ]. Qualitative interview studies addressing the prescribing of antibiotics in uncomplicated upper respiratory tract infections have shown that physicians are aware of the problems of their behaviour in such situations, but the word placebo does not come up [ 15 , 16 ]. However, when asked explicitly about placebo use [ 14 ], physicians seem to accept that such prescriptions can be considered placebo therapy.
The main reason for prescribing antibiotics and other unnecessary treatments is the perceived wish of or pressure from the patient [ 15 – 18 ]. There is some data that physicians overestimate the extent to which patients expect a prescription [ 19 ], suggesting that other, possibly subconscious, reasons might also play a role. Placebo prescription in such a situation is not a case of deception, but a conflict between the professional integrity of the physician and the patient's wish [ 8 ]. Physicians also often raise the issue of remaining uncertainty as a justification [ 15 , 16 ]. For example, a bacterial origin of the infection or a bacterial super-infection cannot be ruled out. However, one could suggest that convenience is often a more important motivation for using a non-indicated treatment than uncertainty. It has been argued that such a use of antibiotics is unethical, unprofessional and harmful [ 8 , 20 ].
In scenario 3, a mother firmly believing in homoeopathic remedies is seeking a paediatrician for her 2-year-old child suffering from symptoms of a common cold. Homoeopathy is a widely used alternative therapy practised both by physicians and non-medical practitioners. Its most controversial aspect is the use of remedies which are prepared in serial dilution steps with vigorous shaking in between (potentization), commonly to the extent that no molecules of the original substance remain. Homoeopaths believe that during the dilution process information passes from the diluted agent to the solvent, which, in the light of current knowledge, seems implausible. Therefore, many scientists are convinced that highly diluted homoeopathic remedies are placebos. As they often do not contain any ‘active substance’ in a chemical sense, they might even qualify as pure placebos. From such a perspective, homoeopathy could be considered a pseudo-therapy.
In our scenario, history and physical examination do not provide any indication for relevant risks but the child clearly suffers from bothersome symptoms. The mother asks for a homoeopathic remedy because the child improved very quickly in a similar situation when another physician prescribed the remedy. The paediatrician is sceptical about homoeopathy but he has seen some astonishing cases, so he is not really certain. Furthermore, he considers the risk minimal. He prescribes the remedy saying that he personally is a bit sceptical about homoeopathy, but it might be worth trying, and if the symptoms deteriorate the mother should return.
Surveys have shown that the use of complementary therapies such as homoeopathy, herbal medicines or vitamins by sceptical physicians is also much more widespread than the use of pure placebos [ 14 , 21 , 22 ]. The motivational pattern for the physician is a mixture of convenience (he wants to respect the mother's wish, and to avoid a conflict or losing a client) and uncertainty (he cannot rule out with certainty that homoeopathy is an active therapy). Scientists would clearly consider this a placebo prescription, but might have diverging views on whether the pragmatic approach of the physician is acceptable.
In scenario 4, the mother and her 2-year old child visit a convinced homoeopath who prescribes a highly diluted homoeopathic remedy. Obviously, for the scientist, homoeopathy remains a placebo (or pseudo-therapy). Instead, based on his daily experience, the homoeopath is convinced that the prescribed remedy is a ‘true’ active treatment. The scientific doubts of researchers not using this therapy are regularly discarded. Patients seeking a homoeopath usually believe that this is or at least could be an active therapy, although some are sceptic.
Surveys on placebo use among physicians do not include questions on this type of placebo use. The reason is obvious: those using the treatment in this manner do not consider it a placebo. As they believe to act in the best interest of their clients, neither do they have any ethical problem. Surveys on the use of often highly controversial complementary and alternative therapies show that they are highly prevalent in many countries [ 23 ]. Some scientists see it as their duty to inform society about the ‘truth’ and consider it as ethically problematic that providers use therapies they consider disproven by science [ 24 , pp. 244–250].
In the last scenario 5, an orthopaedic surgeon performs an arthroscopic débridement in a patient with osteoarthritis of the knee. This is a procedure in which an endoscope is introduced into the arthritic joint. The joint is then lavaged, rough cartilage is shaved and loose debris removed. Surgeons who perform this procedure usually consider it to be an active and effective therapy. Patients are unlikely to undergo this invasive treatment unless they share this view (after probably having been informed in a way supporting this view). However, many scientists consider improvements seen after such a treatment to be a placebo effect, as a rigorous randomized trial did not find any improved outcomes over those of a sham intervention [ 25 ].
The case differs considerably from scenario 4: arthroscopic débridement clearly cannot be considered a pure placebo but is an invasive, intense intervention. Compared with homoeopathic treatment, it is associated with much greater direct risks. Contrary to homoeopaths, surgeons usually claim to practice scientific medicine based on the best available current evidence. To justify their behaviour, they therefore have to question the validity of the relevant study results, at least for the selection of patients in whom they actually perform the procedure, and claim that the way they use the intervention is clearly not a placebo.
If physicians discuss the use of placebo interventions in practice, they typically think of the intentional application of pure placebos (scenario 1). In this classical case providers, scientists and informed patients all agree that this is a placebo intervention. In the remaining scenarios, the situation is far less clear. Most readers would probably agree that scenarios 2 and 3 can be considered examples of placebo interventions as the provider at least to some extent uses the intervention for placebo purposes. Scenario 4 is a typical example of a strong conflict between perspectives. Many readers might have problems in considering scenario 5 a good example of a placebo intervention but according to the best available evidence, the procedure meets the definition by Shapiro and Morris. The scientific perspective summarized in this definition postulates an objective knowledge on what has specific effects and what not. This knowledge is often uncertain and incomplete. Those involved directly in the clinical encounter—physicians and patients—sometimes ignore the scientific perspective. In the discussion on placebo use, these differences in perspectives are often not reflected. This leads to misunderstandings. We suggest that apart from the intentional use of pure placebos (scenario 1), the word placebo interventions should be used more carefully.
3. Investigating whether placebo effects are clinically relevant. the conventional approach
According to Shapiro & Morris, ‘a placebo effect is defined as the psychological or psychophysiological effect produced by placebos’ [ 1 , p. 371]. This questionable view of placebo effects has strongly influenced the approaches for quantifying such effects used in clinical research.
For decades, improvements in placebo groups of randomized clinical trials have been interpreted as evidence for placebo effects. An analysis of the proportion of patients reporting satisfactory relief after receiving placebo in 15 controlled trials published by Beecher in 1955 in JAMA ( The Journal of the American Medical Association ) [ 26 ] is probably the most cited article in the field of placebo research. This article is the basis of a widely quoted myth that the average size of placebo effects is about 35 per cent. Beecher further claimed that the small standard error in his analysis (2.2%) indicates the constancy of the placebo effect. In 1994, a major review published in JAMA claimed even larger placebo effects based on the improvement in placebo groups [ 27 ]. A careful re-analysis of the original studies included in Beecher's review concluded that spontaneous improvement, fluctuation of symptoms, regression to the mean, additional treatment, response biases and misquotation were plausible alternative explanations for the presumed placebo effects [ 28 ]. These reasons also explain why it is almost impossible to reliably judge in routine clinical practice whether a placebo effect has occurred. A recent analysis of trials including both a placebo and no-treatment control group also found relevant improvement in many no-treatment groups [ 29 ]. In conclusion, changes observed in patients receiving placebo over time are not a reliable way to estimate the size of placebo effects.
From a methodological point of view, it seems obvious that for assessing the size of placebo effects, a no-treatment control group is crucial [ 30 ]. However, trials including both a placebo and a no-treatment group are comparably rare and widely dispersed in the medical literature. When, in 2001, the leading medical journal, the New England Journal of Medicine , published a meta-analysis of 114 such trials by Hróbjartsson & Gøtzsche [ 31 ] titled ‘Is the Placebo Powerless?’ this provoked a major debate. In the trials included in this review, placebo, on average, did not have a significant effect over no-treatment when outcomes were binary, regardless of whether these outcomes were subjective or objective. For continuous outcomes, there was a significant effect over no-treatment when the outcome was patient-reported, but not when it was an objective measure. The authors concluded that they had ‘found little evidence in general that placebo had powerful clinical effects’. This meta-analysis has been heavily criticized (e.g. [ 32 – 36 ]) for mixing highly heterogeneous studies with control interventions, which might not always be considered placebo, for including studies in which all study groups including the no-treatment group received basic treatment with potential impact on the outcomes measured, as well as for a variety of other reasons. Furthermore, subsequent analyses have provided evidence that a subset of studies with outcomes regulated by the autonomic nervous system is susceptible to placebo treatments ([ 37 ]; see also [ 38 ]). However, the overall conclusion that available trials including both a placebo and a no-treatment group do not provide convincing evidence for powerful placebo effects in general remains adequate.
Hróbjartsson & Gøtzsche published updated and expanded versions of their review in 2004 [ 39 ] and 2010 [ 40 ]. The current analysis now includes 202 trials. While effect sizes remained similar to those in the first analysis, effects of placebo interventions over no treatment are now statistically significant for both patient- and observer-reported continuous outcomes and for binary outcomes owing to the larger number of included trials. While the evidence that there are placebo effects is stronger now, the authors still conclude that they ‘did not find that placebo interventions have important clinical effects in general’, as the overall effect size is small and the influence of bias unclear [ 40 ].
4. Problems of the conventional approach to assess the clinical relevance of placebo effects
Randomized trials including both a placebo and a no-treatment control group are clearly more appropriate for investigating the size of placebo effects than trials without a no-treatment group. But are they really providing valid evidence on the size of placebo effects in clinical practice? An important methodological problem regarding the reliability of effect estimates is that patients cannot be blinded for comparisons between placebo and no-treatment. This could result in reporting biases (at least in the case of patient-reported subjective outcomes for which the meta-analyses by Hróbjartsson & Gøtzsche provide the most consistent results), differential use of co-interventions and a variety of other biases. This implies that the effect estimates are quite uncertain.
But there is a much more fundamental question: are randomized trials including both a placebo and a no-treatment group truly a valid way to estimate the size of placebo effects in practice? The classical definition by Shapiro & Morris states that placebo effects are produced by placebos. In line with that thinking, one assumes that the difference between the placebo and the no-treatment group in randomized controlled trials (RCTs) is owing to the placebo intervention. But how should an intervention (e.g. a saline injection) produce an effect if it is objectively without a specific effect? [ 3 ] There now seems to exist a consensus among placebo researchers that what we call placebo effects is a heterogeneous class of psychobiological events attributable to the overall therapeutic context [ 41 ]. The placebo intervention by itself should not produce any effect (otherwise it would not be a true placebo); it completes a complex therapeutical situation and thus conveys meaning, influences expectations and possibly triggers conditioned responses or behaviour changes. If this hypothesis is correct, the same placebo intervention should be associated with different placebo effects depending on the context. Furthermore, very different placebos associated with very different contexts (e.g. pharmacological placebo and sham surgery) should regularly produce different placebo effects. The context in an RCT does not reflect any of the scenarios described in the previous section of this paper. Participants in RCTs must be informed in detail about the aims and procedures in a trial (although some studies deviate from this). The motivations of physicians for delivering a placebo differ strongly from normal practice.
In conclusion, the focus on the placebo intervention as the cause of placebo effects is misleading and should be replaced by a focus on the context (including the placebo intervention). However, even if this shift of focus will occur, it seems likely that owing to their specific context situation, RCTs can provide only crude estimators of the size of placebo effects (better, context effects) in routine clinical practice.
5. Moving from research on placebo effects to research on context effects
Apart from the strong evidence from experimental research supporting the contextual interpretation of placebo effects [ 41 , 42 ], there is also increasing evidence from clinical research. The most recent version of Hróbjartsson & Gøtzsche's [ 40 ] review itself found that placebo effects were larger for physical placebos (compared with pharmacological or psychological placebos), in trials not informing patients that a placebo intervention was administered, and in trials with the explicit purpose of studying placebo effects [ 40 ]. Meta-analyses of changes over time in placebo groups in trials without a no-treatment control have identified a variety of context factors associated with effects size, too. For example, trials in which placebo was injected subcutaneously reported higher improvement rates than trials using oral placebos [ 43 ]. An elegant randomized trial found that a placebo acupuncture intervention was associated with significantly greater clinical effects when provided in an empathic compared with a neutral manner [ 44 ].
In principle, studies using the open–hidden paradigm could provide important information as to whether perceiving the act of treatment (be it a placebo or an active intervention) makes a difference. In such studies, an active substance and/or a placebo intervention are administered both in an overt and in a covert fashion [ 45 ]. For example, in a study by Benedetti et al . [ 46 ], patients with post-operative pain with an intravenous drip received either no treatment, an open or a hidden injection of saline (placebo) or of the cholecystokinin antagonist proglumide, which is known to potentiate analgesia induced by morphine and endorphins. Pain intensity was similar in patients receiving no treatment or a hidden injection of saline or proglumide, while it decreased after open injection of saline and even more after proglumide. These results indicate that both saline and proglumide do not have any direct (specific) analgesic effect, but that an overt injection is associated with reduced pain. The results further suggest that proglumide potentiates a placebo-activated endogenous opioid system if applied in an open manner. While the open–hidden paradigm is a fascinating approach, it is, however, not feasible for most treatments as a hidden administration is not possible.
There are a variety of further approaches to investigate the influence of context factors. If we assume that context factors can modify the clinical response to both placebo and active treatment, this can be investigated directly in randomized trials. For example, trials using a balanced placebo design investigate simultaneously the influence of a specific (e.g. drug versus placebo) and a non-specific or context factor (e.g. positive or neutral information). Such trials are infrequent; however, the available studies suggest that context factors not only have direct effects but also interact with specific effects by either increasing or decreasing the differences between active treatment and placebo [ 47 , 48 ].
If we assume that all healthcare interventions can be associated with context effects and that (as we hope) the majority of interventions have specific activity, the majority of context effects in clinical practice should be associated with active treatments. Obviously, context factors can be and have been investigated directly in randomized trials without manipulating the active treatment. Again a systematic review suggests that context factor matters [ 49 ], but owing to the relatively small number of studies and lack of replications the evidence base is relatively weak. There is considerable research on ‘specific’ context factors such as expectations [ 50 , 51 ] or empathy [ 52 ]. However, in our view this should not be called placebo research. What is often described as harnessing placebo effects might be better summarized as harnessing context effects or as attempts to create optimal healing environments [ 53 , 54 ].
6. Bad placebo interventions and good context effects
While we do not have sound evidence regarding how relevant they are in clinical practice, there is a common belief that good physicians should harness placebo and/or context effects to maximize the benefits to their patients [ 55 – 57 ]—however, the use of placebo interventions should be avoided unless absolutely necessary [ 58 ]. A qualitative study by Comaroff published in 1976 [ 17 ] provided interesting insights into why this is the case. For this study, the views of 51 general practitioners on placebo therapy were elicited indirectly, in the context of a more general discussion about prescribing behaviour. Practitioners were first asked to estimate the proportion of their consultations which culminated in prescribing a treatment. All participants set the proportion at 70 per cent or above. In their elaborate answers, most practitioners spontaneously stated that they did not consider all prescriptions as truly necessary and felt necessitated to provide justifications. Implicitly, the answers clearly indicated that the physicians had internalized a professional ideal, which requires that any treatment should be specific in effect and administered or prescribed only when necessary. However, this ideal conflicted with the realities of general practitioners in the real world. Seeing only unselected patients, general practitioners faced considerable uncertainty but still needed to make decisions. Making a firm diagnosis in general practice was often impossible or unnecessary, implying that the basis for choosing a specific treatment was weak. On the other hand, physicians usually believed that patients expected a clear diagnosis and a treatment. Therefore, the general practitioners often prescribed treatment which could be considered a placebo.
If the professional imperative of specific and necessary treatment is taken seriously, giving a placebo is nothing else than a therapeutic defeat. The physician fails. Harnessing context or placebo effects is only legitimate if associated with a specific treatment. Therefore, intentional use of pure placebo is usually restricted to exceptional situations. When applying (what scientists call) impure placebos, physicians more or less use conscious rationalization strategies to cope with their dilemma. Apart from perceived demand or expectations of patients, important rationalizations are beliefs in the specific activity of the treatment provided, in spite of conflicting evidence, and arguing for avoidance of potential complications [ 18 ].
The available evidence suggests that the use of impure placebos is more frequent in primary care than in specialized care [ 4 ]. This seems plausible as diagnostic uncertainty is higher in unselected patient populations where the number of potential diagnoses is high and the prevalence of each single disease is low. However, uncertainty also occurs frequently in specialized settings.
7. Summary and conclusions
In summary, it is often unclear in the clinical setting as to what is a placebo intervention. This does not apply to the intentional use of pure placebos, but such interventions are infrequently used (although the total number of such uses on a population level still might be a cause for concern). Pseudo-treatments, disproven or non-indicated treatments are used much more frequently, but whether they are considered placebos is often a matter of perspective. What are summarized under the term placebo effects are highly heterogeneous phenomena related to the overall context of healthcare interventions. Calling context effects associated with the application of active interventions placebo effects leads to confusion and should be, in our opinion, avoided. We do not know how large placebo effects actually are in clinical practice, but the available evidence suggests that, on average, they are often small. Because of the professional ideal that all treatments used should be specific in action and used when only necessary, the use of placebo interventions is problematic, while harnessing context effects is clearly legitimate when the treatment is active.
There is a clear need for more research on the role of placebo interventions and the relevance of context effects in clinical practice. This research must take the perspectives of providers and patients into account. Qualitative research can provide important insights into why and how physicians use pure and impure placebos. Investigating the relevance of placebo and context effects for clinical practice will remain a challenge. Studies using the open–hidden approach or a balanced placebo design seem particularly desirable. However, the first approach is rarely possible in clinical practice and the second is expensive. As in a clinical environment many factors cannot be controlled and as effects are likely to be small to modest, such studies need large sample sizes. A promising strategy could be to integrate minor manipulations of context factors (for example, using different communication styles) into randomized trials, which are performed for other purposes. If this is done in a larger number of trials, effects could be investigated with sufficient power in meta-analyses (see also [ 59 ]). However, as the context in studies and routine practice differs, uncertainty will remain regarding the size of context effects in clinical practice.
We think that the professional imperative of specific and necessary treatment is adequate. It is an important basis for the quality and authority of medicine and other acknowledged healthcare professions. However, we also think that the amount of uncertainty in medical practice and its consequences on treatment decisions should be discussed more openly. Downplaying the degree of uncertainty and not accepting that the ideal of specific and necessary treatment often cannot be realized pushes healthcare professionals to use questionable rationalization strategies. It should also be accepted that humans very often behave irrationally and that rituals, myths, seemingly plausible explanations, etc., can strongly affect humans, sometimes even on a somatic level. If uncertainty and irrationality are accepted, we believe that there can be ethically, professionally and scientifically acceptable ways for a limited use of impure placebos (provided that they are associated with very low risks) and exceptional use of pure placebos.
One contribution of 17 to a Theme Issue ‘ Placebo effects in medicine: mechanisms and clinical implications ’.
- 1. Shapiro A. K., Morris L. A. 1978. The placebo effect in medical and psychological therapies. In Handbook of psychotherapy and behavior change (eds Garfield S. L., Bergin A. E.), pp. 369–410 New York, NY: Wiley [ Google Scholar ]
- 2. Grünbaum A. 1986. The placebo concept in medicine and psychiatry. Psychol. Med. 16, 19–38 10.1017/S0033291700002506 ( doi:10.1017/S0033291700002506 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Moerman D. E., Jonas W. B. 2002. Deconstructing the placebo effect and finding the meaning response. Ann. Intern. Med. 136, 471–476 [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Fässler M., Meissner K., Schneider A., Linde K. 2010. Frequency and circumstances of placebo use in clinical practice—a systematic review of empirical studies. BMC Med. 8, 15. 10.1186/1741-7015-8-15 ( doi:10.1186/1741-7015-8-15 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Rich B. A. 2003. A placebo for the pain: a medico-legal case analysis. Pain Med. 4, 366–372 10.1111/j.1526-4637.2003.03046.x ( doi:10.1111/j.1526-4637.2003.03046.x ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Lynoe N., Mattsson B., Sandlund M. 1993. The attitudes of patients and physicians towards placebo treatment—a comparative study. Soc. Sci. Med. 36, 767–774 10.1016/0277-9536(93)90037-5 ( doi:10.1016/0277-9536(93)90037-5 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Foddy B. 2009. A duty to deceive: placebos in clinical practice. Am. J. Bioethics 9, 4–12 10.1080/15265160903318350 ( doi:10.1080/15265160903318350 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Hróbjartsson A. 2008. Clinical placebo interventions are unethical, unnecessary, and unprofessional. J. Clin. Ethics 19, 66–69 [ PubMed ] [ Google Scholar ]
- 9. Goodwin J. S., Goodwin J. M., Vogel A. V. 1979. Knowledge and use of placebos by house officers and nurses. Ann. Intern. Med. 91, 106–110 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Gray G., Flynn P. 1981. A survey of placebo use in a general hospital. Gen. Hosp. Psychiatry 3, 199–203 10.1016/0163-8343(81)90002-5 ( doi:10.1016/0163-8343(81)90002-5 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Hróbjartsson A., Norup M. 2003. The use of placebo interventions in medical practice—a national questionnaire survey of Danish clinicians. Eval. Health Prof. 26, 153–165 10.1177/0163278703026002002 ( doi:10.1177/0163278703026002002 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Sherman R., Hickner J. 2007. Academic physicians use placebos in clinical practice and believe in the mind–body connection. J. Gen. Intern. Med. 23, 7–10 10.1007/s11606-007-0332-z ( doi:10.1007/s11606-007-0332-z ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Fässler M., Gnädinger M., Rosemann T., Biller-Andorno N. 2009. Use of placebo interventions among Swiss primary care providers. BMC Health Serv. Res. 9, 144. 10.1186/1472-6963-9-144 ( doi:10.1186/1472-6963-9-144 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Tilburt J. C., Emanuel E., Kaptchuk T. J., Curlin F. A., Miller F. G. 2008. Prescribing ‘placebo treatments’: results of national survey of US internists and rheumatologists. Br. Med. J. 337, a1938. 10.1136/bmj.a1938 ( doi:10.1136/bmj.a1938 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Butler C. C., Rollnick S., Pill R., Maggs-Rapport F., Stott N. 1998. Understanding the culture of prescribing: qualitative study of general practitioners' and patients' perceptions of antibiotics for sore throats. Br. Med. J. 317, 637–642 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Kumar S., Little P., Britten N. 2003. Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. Br. Med. J. 326, 138. 10.1136/bmj.326.7381.138 ( doi:10.1136/bmj.326.7381.138 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Comaroff J. 1976. A bitter pill to swallow: placebo therapy in general practice. Sociol. Rev. 24, 79–96 [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Schwartz R. K., Soumerai S. B., Avorn J. 1989. Physician motivations for nonscientific drug prescribing. Soc. Sci. Med. 28, 577–582 10.1016/0277-9536(89)90252-9 ( doi:10.1016/0277-9536(89)90252-9 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Lado E., Vacariza M., Fernandez-Gonzalez C., Gestal-Otero J. J., Figueras A. 2008. Influence exerted on drug prescribing by patients' attitudes and expectations and by doctors' perception of such expectations: a cohort and nested case–control study. J. Eval. Clin. Pract. 14, 453–459 10.1111/j.1365-2753.2007.00901.x ( doi:10.1111/j.1365-2753.2007.00901.x ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Miller F. G., Colloca L. 2009. The legitimacy of placebo treatments in clinical practice: evidence and ethics. Am. J. Bioethics 9, 39–47 10.1080/15265160903316263 ( doi:10.1080/15265160903316263 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Meissner K., Höfner L., Linde K. 2010. Häufigkeiten und Gründe für den Einsatz von Placebointerventionen in der allgemeinmedizinsichen Praxis: Erste Ergebnisse einer Fragebogenstudie. Zeitschrift für Allgemeinmedizin 86(suppl.), 97 [ Google Scholar ]
- 22. Classen W., Feingold E. 1983. Use of placebos in medical practice. Pharmacopsychiatry 18, 131–132 10.1055/s-2007-1017341 ( doi:10.1055/s-2007-1017341 ) [ DOI ] [ Google Scholar ]
- 23. Ong C. K., Bodeker G., Burford G., Grundy C., Shein K. 2005. WHO global atlas of traditional, complementary and alternative medicine. Kobe, Japan: World Health Organization [ Google Scholar ]
- 24. Singh S., Ernst E. 2008. Trick or treatment. Alternative medicine on trial. London, UK: Bantam Press [ Google Scholar ]
- 25. Moseley J. B., O'Malley K., Petersen N. J., Menke T. J., Brody B. A., Kuykendall D. H., Hollinsworth J. C., Ashton C. M., Wray N. P. 2002. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N. Engl. J. Med. 347, 81–88 10.1056/NEJMoa013259 ( doi:10.1056/NEJMoa013259 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Beecher H. K. 1955. The powerful placebo. JAMA 159, 1602–1606 [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Turner J. A., Deyo R. A., Loeser J. D., VonKorff M., Fordyce W. E. 1994. The importance of placebo effects in pain treatment and research. JAMA 271, 1609–1614 10.1001/jama.271.20.1609 ( doi:10.1001/jama.271.20.1609 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Kienle G. S., Kiene H. 1997. The powerful placebo effect: fact or fiction? J. Clin. Epidemiol. 50, 1311–1318 10.1016/S0895-4356(97)00203-5 ( doi:10.1016/S0895-4356(97)00203-5 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Krogsboll L. T., Hróbjartsson A., Gotzsche P. C. 2009. Spontaneous improvement in randomised clinical trials: meta-analysis of three-armed trials comparing no treatment, placebo and active intervention. BMC Med. Res. Methodol. 9, 1. 10.1186/1471-2288-9-1 ( doi:10.1186/1471-2288-9-1 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Ernst E., Resch K. L. 1995. Concept of true and perceived placebo effects. Br. Med. J. 311, 551–553 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Hróbjartsson A., Gøtzsche P. C. 2001. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N. Engl. J. Med. 344, 1594–1602 10.1056/NEJM200105243442106 ( doi:10.1056/NEJM200105243442106 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Miller F. G. 2001. Is the placebo powerless? N. Engl. J. Med. 345, 1277. [ PubMed ] [ Google Scholar ]
- 33. Lilford R. J., Braunholtz D. A. 2001. Is the placebo powerless? N. Engl. J. Med. 345, 1277–1278 [ PubMed ] [ Google Scholar ]
- 34. Spiegel D., Kraemer H., Carlson R. W. 2001. Is the placebo powerless? N. Engl. J. Med. 345, 1276. [ PubMed ] [ Google Scholar ]
- 35. McDonald C. J. 2001. Is the placebo powerless? N. Engl. J. Med. 345, 1276–1277 [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Wampold B. E., Minami T., Tierney S. C., Baskin T. W., Bhati K. S. 2005. The placebo is powerful: estimating placebo effects in medicine and psychotherapy from randomized clinical trials. J. Clin. Psychol. 61, 835–854 10.1002/jclp.20129 ( doi:10.1002/jclp.20129 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Meissner K., Distel H., Mitzdorf U. 2007. Evidence for placebo effects on physical but not on biochemical outcome parameters: a review of clinical trials. BMC Med. 5, 3. 10.1186/1741-7015-5-3 ( doi:10.1186/1741-7015-5-3 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Meissner K. 2011. The placebo effect and the autonomic nervous system: evidence for an intimate relationship. Phil. Trans. R. Soc. B 366, 1808–1817 10.1098/rstb.2010.0403 ( doi:10.1098/rstb.2010.0403 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Hróbjartsson A., Gøtzsche P. C. 2004. Placebo interventions for all clinical conditions. Cochrane Database Syst. Rev. 3, CD003974. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Hróbjartsson A., Gøtzsche P. C. 2010. Placebo interventions for all clinical conditions. Cochrane Database Syst. Rev. 1, CD003974. 10.1002/14651858.CD003974.pub3 ( doi:10.1002/14651858.CD003974.pub3 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Finniss D. G., Kaptchuk T. J., Miller F., Benedetti F. 2010. Biological, clinical, and ethical advances of placebo effects. Lancet 375, 686–695 10.1016/S0140-6736(09)61706-2 ( doi:10.1016/S0140-6736(09)61706-2 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Benedetti F. 2008. Placebo effects. Understanding the mechanisms in health and disease. Oxford, UK: Oxford University Press [ Google Scholar ]
- 43. de Craen A. J., Tijssen J. G., de Gans J., Kleijnen J. 2000. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J. Neurol. 247, 183–188 10.1007/s004150050560 ( doi:10.1007/s004150050560 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Kaptchuk T. J., et al. 2008. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. Br. Med. J. 336, 999–1003 10.1136/bmj.39524.439618.25 ( doi:10.1136/bmj.39524.439618.25 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Finniss D. G., Benedetti F. 2005. Mechanisms of the placebo response and their impact on clinical trials and clinical practice. Pain 114, 3–6 10.1016/j.pain.2004.12.012 ( doi:10.1016/j.pain.2004.12.012 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Benedetti F., Amanzio M., Maggi G. 1995. Potentiation of placebo analgesia by proglumide. Lancet 346, 1231. 10.1016/S0140-6736(95)92938-X ( doi:10.1016/S0140-6736(95)92938-X ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Kleijnen J., de Craen A. J., van Everdingen J., Krol L. 1994. Placebo effect in double-blind clinical trials: a review of interactions with medications. Lancet 344, 1347–1349 10.1016/S0140-6736(94)90699-8 ( doi:10.1016/S0140-6736(94)90699-8 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Enck P., Klosterhalfen S., Weimer K., Horing B., Zipfel S. 2011. The placebo response in clinical trials: more questions than answers. Phil. Trans. R. Soc. B 366, 1889–1895 10.1098/rstb.2010.0384 ( doi:10.1098/rstb.2010.0384 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Di Blasi Z., Harkness E., Ernst E., Georgiou A., Kleijnen J. 2001. Influence of context effects on health outcomes: a systematic review. Lancet 357, 757–762 10.1016/S0140-6736(00)04169-6 ( doi:10.1016/S0140-6736(00)04169-6 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Mondloch M. V., Cole D. J., Frank J. W. 2001. Does how you do depend on how you think you'll do? A systematic review of the evidence for a relation between patients' recovery expectations and health outcomes. CMAJ 165, 174–179 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Crow R., Gage H., Hampson S., Hart J., Kimber A., Thomas H. 1999. The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review. Health Technol. Assess. 3, 1–96 [ PubMed ] [ Google Scholar ]
- 52. Pedersen R. 2009. Empirical research on empathy in medicine: a critical review. Patient Educ. Couns. 76, 307–322 10.1016/j.pec.2009.06.012 ( doi:10.1016/j.pec.2009.06.012 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Jonas W. B., Chez R. A., Duffy B., Strand D. 2003. Investigating the impact of optimal healing environments. Altern. Ther. Health Med. 9, 36–40 [ PubMed ] [ Google Scholar ]
- 54. Jonas W. B. 2011. Reframing placebo in research and practice. Phil. Trans. R. Soc. B 366, 1896–1904 10.1098/rstb.2010.0405 ( doi:10.1098/rstb.2010.0405 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Barrett B., Muller D., Rakel D., Rabago D., Marchand L., Scheder J. 2006. Placebo, meaning, and health. Perspect. Biol. Med. 49, 178–198 10.1353/pbm.2006.0019 ( doi:10.1353/pbm.2006.0019 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Benedetti F. 2002. How the doctor's words affect the patient's brain. Eval. Health. Prof. 25, 369–386 10.1177/0163278702238051 ( doi:10.1177/0163278702238051 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Chaput de Saintonge D. M., Herxheimer A. 1994. Harnessing placebo effects in health care. Lancet 344, 995–998 10.1016/S0140-6736(94)91647-0 ( doi:10.1016/S0140-6736(94)91647-0 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Moerman D. E. 2002. The meaning response and the ethics of avoiding placebos. Eval. Health Prof. 25, 399–409 10.1177/0163278702238053 ( doi:10.1177/0163278702238053 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Colloca L., Miller F. G. 2011. Harnessing the placebo effect: the need for translational research. Phil. Trans. R. Soc. B 366, 1922–1930 10.1098/rstb.2010.0399 ( doi:10.1098/rstb.2010.0399 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (180.5 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 01 March 2013
The placebo response in medicine: minimize, maximize or personalize?
- Paul Enck 1 na1 ,
- Ulrike Bingel 2 na1 ,
- Manfred Schedlowski 3 na1 &
- Winfried Rief 4 na1
Nature Reviews Drug Discovery volume 12 , pages 191–204 ( 2013 ) Cite this article
17k Accesses
482 Citations
108 Altmetric
Metrics details
- Clinical pharmacology
- Clinical trials
- Drug discovery and development
- Drug regulation
- Pharmacology
Our understanding of the mechanisms mediating or moderating the placebo response to medicines has grown substantially over the past decade and offers the opportunity to capitalize on its benefits in future drug development as well as in clinical practice. In this article, we discuss three strategies that could be used to modulate the placebo response, depending on which stage of the drug development process they are applied. In clinical trials the placebo effect should be minimized to optimize drug–placebo differences, thus ensuring that the efficacy of the investigational drug can be truly evaluated. Once the drug is approved and in clinical use, placebo effects should be maximized by harnessing patients' expectations and learning mechanisms to improve treatment outcomes. Finally, personalizing placebo responses — which involves considering an individual's genetic predisposition, personality, past medical history and treatment experience — could also maximize therapeutic outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
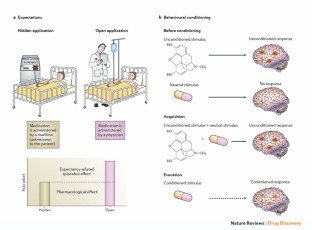
Similar content being viewed by others
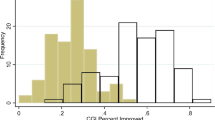
Placebo and nocebo responses in randomised, controlled trials of medications for ADHD: a systematic review and meta-analysis
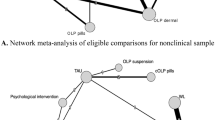
The roles of expectation, comparator, administration route, and population in open-label placebo effects: a network meta-analysis
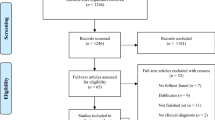
Effects of open-label placebos in clinical trials: a systematic review and meta-analysis
Bingel, U. et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci. Transl. Med. 3 , 70ra14 (2011).
Article CAS PubMed Google Scholar
Colloca, L. & Benedetti, F. Placebos and painkillers: is mind as real as matter? Nature Rev. Neurosci. 6 , 545–552 (2005).
Article CAS Google Scholar
Doering, B. K. & Rief, W. Utilizing placebo mechanisms for dose reduction in pharmacotherapy. Trends Pharmacol. Sci. 33 , 165–172 (2012).
Kaptchuk, T. J. et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 336 , 999–1003 (2008).
Article PubMed PubMed Central Google Scholar
[No authors listed.] An Audience With... Ted Kaptchuk. Nature Rev. Drug Discov. 7 , 554 (2008).
Benedetti, F. Placebo Effects: Understanding the mechanisms in health and disease (Oxford Univ. Press, 2008).
Book Google Scholar
Fields, H. State-dependent opioid control of pain. Nature Rev. Neurosci. 5 , 565–575 (2004).
Millan, M. J. Descending control of pain. Prog. Neurobiol. 66 , 355–474 (2002).
Eippert, F., Finsterbusch, J., Bingel, U. & Buchel, C. Direct evidence for spinal cord involvement in placebo analgesia. Science 326 , 404 (2009).
Amanzio, M. & Benedetti, F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J. Neurosci. 19 , 484–494 (1999).
Article CAS PubMed PubMed Central Google Scholar
Benedetti, F., Amanzio, M., Rosato, R. & Blanchard, C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nature Med. 17 , 1228–1230 (2011).
Eippert, F. et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63 , 533–543 (2009).
Wager, T. D., Scott, D. J. & Zubieta, J. K. Placebo effects on human μ-opioid activity during pain. Proc. Natl Acad. Sci. USA 104 , 11056–11061 (2007).
Guo, J. Y., Wang, J. Y. & Luo, F. Dissection of placebo analgesia in mice: the conditions for activation of opioid and non-opioid systems. J. Psychopharmacol. 24 , 1561–1567 (2010).
Levine, J. D., Gordon, N. C. & Fields, H. L. The mechanism of placebo analgesia. Lancet 2 , 654–657 (1978).
Tracey, I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nature Med. 16 , 1277–1283 (2010).
de la Fuente-Fernandez, R. et al. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science 293 , 1164–1166 (2001).
Lidstone, S. C. et al. Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch. Gen. Psychiatry 67 , 857–865 (2010).
Leuchter, A. F., Cook, I. A., Witte, E. A., Morgan, M. & Abrams, M. Changes in brain function of depressed subjects during treatment with placebo. Am. J. Psychiatry 159 , 122–129 (2002).
Article PubMed Google Scholar
Mayberg, H. S. et al. The functional neuroanatomy of the placebo effect. Am. J. Psychiatry 159 , 728–737 (2002).
Petrovic, P. et al. Placebo in emotional processing-induced expectations of anxiety relief activate a generalized modulatory network. Neuron 46 , 957–969 (2005).
Furmark, T. et al. A link between serotonin-related gene polymorphisms, amygdala activity, and placebo-induced relief from social anxiety. J. Neurosci. 28 , 13066–13074 (2008).
Ader, R. & Cohen, N. Behaviorally conditioned immunosuppression. Psychosom. Med. 37 , 333–340 (1975).
Goebel, M. U. et al. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 16 , 1869–1873 (2002).
Goebel, M. U., Meykadeh, N., Kou, W., Schedlowski, M. & Hengge, U. R. Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychother. Psychosom. 77 , 227–234 (2008).
Schedlowski, M. & Pacheco-Lopez, G. The learned immune response: Pavlov and beyond. Brain Behav. Immun. 24 , 176–185 (2010).
Wirth, T. et al. Repeated recall of learned immunosuppression: evidence from rats and men. Brain Behav. Immun. 25 , 1444–1451 (2011).
Benedetti, F. et al. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J. Neurosci. 23 , 4315–4323 (2003).
Sabbioni, M. E. et al. Classically conditioned changes in plasma cortisol levels induced by dexamethasone in healthy men. FASEB J. 11 , 1291–1296 (1997).
Benedetti, F. The Patient's Brain: The neuroscience behind the doctor–patient relationship (Oxford Univ. Press, 2011).
Google Scholar
Rief, W., Bingel, U., Schedlowski, M. & Enck, P. Mechanisms involved in placebo and nocebo responses and implications for drug trials. Clin. Pharmacol. Ther. 90 , 722–726 (2011).
Hill, A. B. Suspended judgement. Memories of the British streptomycin trial in tuberculosis. The first randomized clinical trial. Control. Clin. Trials 11 , 77–79 (1990).
Diener, H. C., Dowson, A. J., Ferrari, M., Nappi, G. & Tfelt-Hansen, P. Unbalanced randomization influences placebo response: scientific versus ethical issues around the use of placebo in migraine trials. Cephalalgia 19 , 699–700 (1999).
Papakostas, G. I. & Fava, M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur. Neuropsychopharmacol. 19 , 34–40 (2009).
Mallinckrodt, C. H., Zhang, L., Prucka, W. R. & Millen, B. A. Signal detection and placebo response in schizophrenia: parallels with depression. Psychopharmacol. Bull. 43 , 53–72 (2010).
PubMed Google Scholar
Sinyor, M. et al. Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. J. Clin. Psychiatry 71 , 270–279 (2010).
Nakamura, Y. et al. Investigating dose-dependent effects of placebo analgesia: a psychophysiological approach. Pain 153 , 227–237 (2012).
Rutherford, B. R., Sneed, J. R. & Roose, S. P. Does study design influence outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychother. Psychosom. 78 , 172–181 (2009).
Quigley, E. M. et al. Randomised clinical trials: linaclotide phase 3 studies in IBS-C — a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment. Pharmacol. Ther. 37 , 49–61 (2013).
Staskin, D. R., Michel, M. C., Sun, F., Guan, Z. & Morrow, J. D. The effect of elective sham dose escalation on the placebo response during an antimuscarinic trial for overactive bladder symptoms. J. Urol. 187 , 1721–1726 (2012).
Mallinckrodt, C. et al. A case study comparing a randomized withdrawal trial and a double-blind long-term trial for assessing the long-term efficacy of an antidepressant. Pharm. Stat. 6 , 9–22 (2007).
Suchman, A. L. & Ader, R. Classic conditioning and placebo effects in crossover studies. Clin. Pharmacol. Ther. 52 , 372–377 (1992).
Hrobjartsson, A. & Boutron, I. Blinding in randomized clinical trials: imposed impartiality. Clin. Pharmacol. Ther. 90 , 732–736 (2011).
Moncrieff, J., Wessely, S. & Hardy, R. Active placebos versus antidepressants for depression. Cochrane Database Syst. Rev. 2004 , CD003012 (2004).
PubMed Central Google Scholar
Rief, W. & Glombiewski, J. A. The hidden effects of blinded, placebo controlled randomized trials: an experimental investigation. Pain 153 , 2473–2477 (2012).
Toth, C. et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain 153 , 2073–2082 (2012).
de la Fuente-Fernandez, R. The powerful pre-treatment effect: placebo responses in restless legs syndrome trials. Eur. J. Neurol. 19 , 1305–1310 (2012).
Tedeschini, E., Fava, M., Goodness, T. M. & Papakostas, G. I. Relationship between probability of receiving placebo and probability of prematurely discontinuing treatment in double-blind, randomized clinical trials for MDD: a meta-analysis. Eur. Neuropsychopharmacol. 20 , 562–567 (2010).
Zelen, M. A new design for randomized clinical trials. N. Engl. J. Med. 300 , 1242–1245 (1979).
Kirsch, I. Are drug and placebo effects in depression additive? Biol. Psychiatry 47 , 733–735 (2000).
Muthen, B. & Brown, H. C. Estimating drug effects in the presence of placebo response: causal inference using growth mixture modeling. Stat. Med. 28 , 3363–3385 (2009).
Dworkin, R. H. et al. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain 153 , 1148–1158 (2012).
US Food and Drug Administration (FDA). Guidance for Industry: E 10 Choice of control group and related issues in clinical trials. FDA website [online] , (2001).
Mulla, S. M., Scott, I. A., Jackevicius, C. A., You, J. J. & Guyatt, G. H. How to use a noninferiority trial: users' guides to the medical literature. JAMA 308 , 2605–2611 (2012).
Woods, S. W., Gueorguieva, R. V., Baker, C. B. & Makuch, R. W. Control group bias in randomized atypical antipsychotic medication trials for schizophrenia. Arch. Gen. Psychiatry 62 , 961–970 (2005).
Estellat, C. & Ravaud, P. Lack of head-to-head trials and fair control arms: randomized controlled trials of biologic treatment for rheumatoid arthritis. Arch. Intern. Med. 172 , 237–244 (2012).
Huitfeldt, B., Hummel, J. & the European Federation of Statisticians in the Pharmaceutical Industry (EFSPI). The draft FDA guideline on non-inferiority clinical trials: a critical review from European pharmaceutical industry statisticians. Pharm. Stat. 10 , 414–419 (2011).
Leon, A. C. Comparative effectiveness clinical trials in psychiatry: superiority, noninferiority, and the role of active comparators. J. Clin. Psychiatry 72 , 1344–1349 (2011).
Rief, W. et al. Meta-analysis of the placebo response in antidepressant trials. J. Affect. Disord. 118 , 1–8 (2009).
Hrobjartsson, A. & Gotzsche, P. C. Placebo interventions for all clinical conditions. Cochrane Database Syst. Rev. 2010 , CD003974 (2010).
Kirsch, I. et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 5 , e45 (2008).
Nijs, J. et al. Recruitment bias in chronic pain research: whiplash as a model. Clin. Rheumatol. 30 , 1481–1489 (2011).
Kobak, K. A., Kane, J. M., Thase, M. E. & Nierenberg, A. A. Why do clinical trials fail? The problem of measurement error in clinical trials: time to test new paradigms? J. Clin. Psychopharmacol. 27 , 1–5 (2007).
Pressman, A., Avins, A. L., Neuhaus, J., Ackerson, L. & Rudd, P. Adherence to placebo and mortality in the Beta Blocker Evaluation of Survival Trial (BEST). Contemp. Clin. Trials 33 , 492–498 (2012).
Boehmer, J. & Yong, P. How well does blinding work in randomized controlled trials?: a counterpoint. Clin. Pharmacol. Ther. 85 , 463–465 (2009).
Cepeda, M. S., Berlin, J. A., Gao, C. Y., Wiegand, F. & Wada, D. R. Placebo response changes depending on the neuropathic pain syndrome: results of a systematic review and meta-analysis. Pain Med. 13 , 575–595 (2012).
Enck, P., Klosterhalfen, S. & Zipfel, S. Novel study designs to investigate the placebo response. BMC Med. Res. Methodol. 11 , 90 (2011).
Colloca, L., Lopiano, L., Lanotte, M. & Benedetti, F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet Neurol. 3 , 679–684 (2004).
Enck, P., Grundy, D. & Klosterhalfen, S. A novel placebo-controlled clinical study design without ethical concerns — the free choice paradigm. Med. Hypotheses 79 , 880–882 (2012).
O'Neil, C. C. & Miller, F. G. When scientists deceive: applying the federal regulations. J. Law Med. Eth. 37 , 344–350 (2009).
Article Google Scholar
Fässler, M., Meissner, K., Schneider, A. & Linde, K. Frequency and circumstances of placebo use in clinical practice — a systematic review of empirical studies. BMC Med. 8 , 15 (2010).
Miller, F. G. & Colloca, L. The placebo phenomenon and medical ethics: rethinking the relationship between informed consent and risk–benefit assessment. Theor. Med. Bioeth. 32 , 29–43 (2011).
Kaptchuk, T. J. et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS ONE 5 , e15591 (2010).
Juergens, M. C., Seekatz, B., Moosdorf, R. G., Petrie, K. J. & Rief, W. Illness beliefs before cardiac surgery predict disability, quality of life, and depression 3 months later. J. Psychosom. Res. 68 , 553–560 (2010).
Petrie, K. J., Cameron, L. D., Ellis, C. J., Buick, D. & Weinman, J. Changing illness perceptions after myocardial infarction: an early intervention randomized controlled trial. Psychosom. Med. 64 , 580–586 (2002).
Barefoot, J. C. et al. Recovery expectations and long-term prognosis of patients with coronary heart disease. Arch. Intern. Med. 171 , 929–935 (2011).
Judge, A. et al. Pre-operative expectation predicts 12-month post-operative outcome among patients undergoing primary total hip replacement in European orthopaedic centres. Osteoarthritis Cartilage 19 , 659–667 (2011).
Mercado, R. et al. Expectation and the placebo effect in Parkinson's disease patients with subthalamic nucleus deep brain stimulation. Mov. Disord. 21 , 1457–1461 (2006).
Chen, J. A. et al. Association between patient beliefs regarding assigned treatment and clinical response: reanalysis of data from the Hypericum Depression Trial Study Group. J. Clin. Psychiat. 72 , 1669–1676 (2011).
Bodenheimer, T. The future of primary care: transforming practice. N. Engl. J. Med. 359 , 2086–2089 (2008).
Acosta, J. I., Thiel, K. J., Sanabria, F., Browning, J. R. & Neisewander, J. L. Effect of schedule of reinforcement on cue-elicited reinstatement of cocaine-seeking behavior. Behav. Pharmacol. 19 , 129–136 (2008).
Ader, R. et al. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom. Med. 72 , 192–197 (2010).
Sandler, A. D., Glesne, C. E. & Bodfish, J. W. Conditioned placebo dose reduction: a new treatment in attention-deficit hyperactivity disorder? J. Dev. Behav. Pediatr. 31 , 369–375 (2010).
Ader, R. in The Placebo Effect: An Interdisciplinary Exploration (ed. Harrington, A.) 138–165 (Harvard Univ. Press, 1997).
Hadamitzky, M., Engler, H. & Schedlowski, M. Learned immunosuppression: extinction, renewal, and the challenge of reconsolidation. J. Neuroimmune Pharmacol. 13 Jul 2012 (doi:10.1007/s11481-012-9388-6).
Koudriavtseva, T., Onesti, E., Pestalozza, I. F., Sperduti, I. & Jandolo, B. The importance of physician–patient relationship for improvement of adherence to long-term therapy: data of survey in a cohort of multiple sclerosis patients with mild and moderate disability. Neurol. Sci. 33 , 575–584 (2012).
Schneider, R. & Kuhl, J. Placebo forte: ways to maximize unspecific treatment effects. Med. Hypotheses 78 , 744–751 (2012).
Waber, R. L., Shiv, B., Carmon, Z. & Ariely, D. Commercial features of placebo and therapeutic efficacy. JAMA 299 , 1016–1017 (2008).
Linde, K. et al. Acupuncture for patients with migraine: a randomized controlled trial. JAMA 293 , 2118–2125 (2005).
Ober, K. et al. Plasma noradrenaline and state anxiety levels predict placebo response in learned immunosuppression. Clin. Pharmacol. Ther. 91 , 220–226 (2012).
Wager, T. D., Atlas, L. Y., Leotti, L. A. & Rilling, J. K. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J. Neurosci. 31 , 439–452 (2011).
Stein, N., Sprenger, C., Scholz, J., Wiech, K. & Bingel, U. White matter integrity of the descending pain modulatory system is associated with inter-individual differences in placebo analgesia. Pain 153 , 2210–2217 (2012).
Benedetti, F. et al. Loss of expectation-related mechanisms in Alzheimer's disease makes analgesic therapies less effective. Pain 121 , 133–144 (2006).
Baeken, C., Vanderhasselt, M. A. & De Raedt, R. Baseline 'state anxiety' influences HPA-axis sensitivity to one sham-controlled HF-rTMS session applied to the right dorsolateral prefrontal cortex. Psychoneuroendocrinology 36 , 60–67 (2011).
Lyby, P. S., Aslaksen, P. M. & Flaten, M. A. Variability in placebo analgesia and the role of fear of pain — an ERP study. Pain 152 , 2405–2412 (2011).
Flaten, M. A., Aslaksen, P. M., Lyby, P. S. & Bjorkedal, E. The relation of emotions to placebo responses. Phil. Trans. R. Soc. B 366 , 1818–1827 (2011).
Linde, K. et al. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain 128 , 264–271 (2007).
Flaten, M. A., Aslaksen, P. M., Finset, A., Simonsen, T. & Johansen, O. Cognitive and emotional factors in placebo analgesia. J. Psychosom. Res. 61 , 81–89 (2006).
Geers, A. L., Helfer, S. G., Kosbab, K., Weiland, P. E. & Landry, S. J. Reconsidering the role of personality in placebo effects: dispositional optimism, situational expectations, and the placebo response. J. Psychosom. Res. 58 , 121–127 (2005).
Morton, D. L., Watson, A., El-Deredy, W. & Jones, A. K. Reproducibility of placebo analgesia: effect of dispositional optimism. Pain 146 , 194–198 (2009).
Geers, A. L., Wellman, J. A., Fowler, S. L., Helfer, S. G. & France, C. R. Dispositional optimism predicts placebo analgesia. J. Pain. 11 , 1165–1171 (2010).
De Pascalis, V., Chiaradia, C. & Carotenuto, E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain 96 , 393–402 (2002).
Reynaert, C., Janne, P., Vause, M., Zdanowicz, N. & Lejeune, D. Clinical trials of antidepressants: the hidden face: where locus of control appears to play a key role in depression outcome. Psychopharmacology 119 , 449–454 (1995).
Moerman, D. E. Cultural variations in the placebo effect: ulcers, anxiety, and blood pressure. Med. Anthropol. Quarterly 14 , 51–72 (2000).
Schneider, A. et al. Acupuncture treatment in irritable bowel syndrome. Gut 55 , 649–654 (2006).
Lindstedt, F. et al. Conditioned pain modulation is associated with common polymorphisms in the serotonin transporter gene. PLoS ONE 6 , e18252 (2011).
Leuchter, A. F., McCracken, J. T., Hunter, A. M., Cook, I. A. & Alpert, J. E. Monoamine oxidase A and catechol- O -methyltransferase functional polymorphisms and the placebo response in major depressive disorder. J. Clin. Psychopharmacol. 29 , 372–377 (2009).
Kaptchuk, T. J. et al. Do “placebo responders” exist? Contemp. Clin. Trials. 29 , 587–595 (2008).
Whalley, B., Hyland, M. E. & Kirsch, I. Consistency of the placebo effect. J. Psychosom. Res. 64 , 537–541 (2008).
Caponigro, G. & Sellers, W. R. Advances in the preclinical testing of cancer therapeutic hypotheses. Nature Rev. Drug Discov. 10 , 179–187 (2011).
Beckman, R. A., Clark, J. & Chen, C. Integrating predictive biomarkers and classifiers into oncology clinical development programmes. Nature Rev. Drug Discov. 10 , 735–748 (2011).
Colloca, L., Sigaudo, M. & Benedetti, F. The role of learning in nocebo and placebo effects. Pain 136 , 211–218 (2008).
Iovieno, N. & Papakostas, G. I. Does the presence of an open-label antidepressant treatment period influence study outcome in clinical trials examining augmentation/combination strategies in treatment partial responders/nonresponders with major depressive disorder? J. Clin. Psychiatry 73 , 676–683 (2012).
Wechsler, M. E. et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N. Engl. J. Med. 365 , 119–126 (2011).
Finniss, D. G., Kaptchuk, T. J., Miller, F. & Benedetti, F. Biological, clinical, and ethical advances of placebo effects. Lancet 375 , 686–695 (2010).
McRae, C. et al. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch. Gen. Psychiatry 61 , 412–420 (2004).
Moseley, J. B. et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N. Engl. J. Med. 347 , 81–88 (2002).
Quessy, S. N. & Rowbotham, M. C. Placebo response in neuropathic pain trials. Pain 138 , 479–483 (2008).
Khan, A. Redding, N. & Brown, W. A. The persistence of the placebo response in antidepressant clinical trials. J. Psychiatr. Res. 42 , 791–796 (2008).
Colloca, L. & Benedetti, F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr. Opin. Anaesthesiol. 20 , 435–439 (2007).
Colloca, L. & Finniss, D. Nocebo effects, patient–clinician communication, and therapeutic outcomes. JAMA 307 , 567–568 (2012).
Sullivan, R., Behncke, I. & Purushotham, A. Why do we love medicines so much? An evolutionary perspective on the human love of pills, potions and placebo. EMBO Rep. 11 , 572–578 (2010).
Volkow, N. D. et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J. Neurosci. 23 , 11461–11468 (2003).
Benedetti, F., Amanzio, M., Baldi, S., Casadio, C. & Maggi, G. Inducing placebo respiratory depressant responses in humans via opioid receptors. Eur. J. Neurosci. 11 , 625–631 (1999).
Isenberg, S. A., Lehrer, P. M. & Hochron, S. The effects of suggestion and emotional arousal on pulmonary function in asthma — a review and a hypothesis regarding vagal mediation. Psychosom. Med. 54 , 192–216 (1992).
Pollo, A., Vighetti, S., Rainero, I. & Benedetti, F. Placebo analgesia and the heart. Pain 102 , 125–133 (2003).
Ronel, J. et al. Effects of verbal suggestion on coronary arteries: results of a randomized controlled experimental investigation during coronary angiography. Am. Heart J. 162 , 507–511 (2011).
Amigo, I., Cuesta, V., Fernandez, A. & Gonzalez, A. The effect of verbal instructions on blood pressure measurement. J. Hypertens. 11 , 293–296 (1993).
Lanotte, M. et al. Expectation enhances autonomic responses to stimulation of the human subthalamic limbic region. Brain Behav. Immun. 19 , 500–509 (2005).
Elsenbruch, S. et al. Neural mechanisms mediating the effects of expectation in visceral placebo analgesia: an fMRI study in healthy placebo responders and non-responders. Pain 153 , 382–390 (2012).
Price, D. D., Craggs, J., Verne, G. N., Perlstein, W. M. & Robinson, M. E. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain 127 , 63–72 (2007).
Meissner, K. Effects of placebo interventions on gastric motility and general autonomic activity. J. Psychosom. Res. 66 , 391–398 (2009).
Scott, D. J. et al. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 55 , 325–336 (2007).
Scott, D. J. et al. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry 65 , 220–231 (2008).
Hall, K. T. et al. Catechol- O -methyltransferase Val158Met polymorphism predicts placebo effect in irritable bowel syndrome. PLoS ONE 7 , e48135 (2012).
Benedetti, F. et al. Electrophysiological properties of thalamic, subthalamic and nigral neurons during the anti-parkinsonian placebo response. J. Physiol. 587 , 3869–3883 (2009).
Potkin, S. et al. Placebo response trajectories in short-term and long-term antipsychotic trials in schizophrenia. Schizophr. Res. 132 , 108–113 (2011).
Lee, S., Walker, J. R., Jakul, L. & Sexton, K. Does elimination of placebo responders in a placebo run-in increase the treatment effect in randomized clinical trials? A meta-analytic evaluation. Depress. Anxiety 19 , 10–19 (2004).
Madsen, L. G. & Bytzer, P. Single subject trials as a research instrument in gastrointestinal pharmacology. Aliment. Pharmacol. Ther. 16 , 189–196 (2002).
Ivanova, A. & Tamura, R. N. A two-way enriched clinical trial design: combining advantages of placebo lead-in and randomized withdrawal. Stat. Methods Med. Res. 4 Dec 2011 (doi:10.1177/0962280211431023).
King, M. et al. Conceptual framework and systematic review of the effects of participants' and professionals' preferences in randomised controlled trials. Health Technol. Assess. 9 , 1–186 (2005).
De Allegri, M. et al. Step-wedge cluster-randomised community-based trials: an application to the study of the impact of community health insurance. Health Res. Policy Syst. 6 , 10 (2008).
Weijer, C. et al. The Ottawa statement on the ethical design and conduct of cluster randomized trials. PLoS Med. 9 , e1001346 (2012).
Mondaini, N. et al. Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J. Sex. Med. 4 , 1708–1712 (2007).
Petrie, K. J. et al. Effect of providing information about normal test results on patients' reassurance: randomised controlled trial. BMJ 334 , 352–353 (2007).
Colloca, L. & Benedetti, F. How prior experience shapes placebo analgesia. Pain 124 , 126–133 (2006).
Eikelboom, R. & Stewart, J. Conditioning of drug-induced physiological responses. Psych. Rev. 89 , 507–528 (1982).
Barsky, A. J., Saintfort, R., Rogers, M. P. & Borus, J. F. Nonspecific medication side effects and the nocebo phenomenon. JAMA 287 , 622–626 (2002).
Preston, R. A., Materson, B. J., Reda, D. J. & Williams, D. W. Placebo-associated blood pressure response and adverse effects in the treatment of hypertension. Arch. Intern. Med. 160 , 1449–1454 (2000).
Rief, W., Avorn, J. & Barsky, A. J. Medication-attributed adverse effects in placebo groups. Implications for assessment of adverse effects. Arch. Intern. Med. 166 , 155–160 (2006).
Rief, W. et al. Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials. A systematic review and meta-analysis. Drug Safety 32 , 1041–1056 (2009).
de la Cruz, M., Hui, D., Parsons, H. A. & Bruera, E. Placebo and nocebo effects in randomized double-blind clinical trials of agents for the therapy for fatigue in patients with advanced cancer. Cancer 116 , 766–774 (2010).
Häuser, W., Bartram, C., Bartram-Wunn, E. & Tolle, T. Adverse events attributable to nocebo in randomized controlled drug trials in fibromyalgia syndrome and painful diabetic peripheral neuropathy: systematic review. Clin. J. Pain 28 , 437–451 (2012).
Amanzio, M., Corazzini, L. L., Vase, L. & Benedetti, F. A systematic review of adverse events in placebo groups of anti-migraine clinical trials. Pain 146 , 261–269 (2009).
Papadopoulos, D. & Mitsikostas, D. D. Nocebo effects in multiple sclerosis trials: a meta-analysis. Mult. Scler. 16 , 816–828 (2010).
Wise, R. A. et al. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J. Allergy Clin. Immunol. 124 , 436–444.e8 (2009).
Koyama, T., McHaffie, J. G., Laurienti, P. J. & Coghill, R. C. The subjective experience of pain: where expectations become reality. Proc. Natl Acad. Sci. USA 102 , 12950–12955 (2005).
Keltner, J. R. et al. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J. Neurosci. 26 , 4437–4443 (2006).
Craig, K. D. & Prkachin, K. M. Social modeling influences on sensory decision theory and psychophysiological indexes of pain. J. Pers. Soc. Psychol. 36 , 805–815 (1978).
Nestoriuc, Y., Orav, E. J., Liang, M. H., Horne, R. & Barsky, A. J. Prediction of nonspecific side effects in rheumatoid arthritis patients by beliefs about medicines. Arthritis Care Res. 62 , 791–799 (2010).
Petrie, K. J. et al. Worries about modernity predict symptom complaints after environmental pesticide spraying. Psychosom. Med. 67 , 778–782 (2005).
Jackevicius, C. A., Mamdani, M. & Tu, J. V. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 288 , 462–467 (2002).
Colagiuri, B., McGuinness, K., Boakes, R. A. & Butow, P. N. Warning about side effects can increase their occurrence: an experimental model using placebo treatment for sleep difficulty. J. Psychopharmacol. 26 , 1540–1547 (2012).
Download references
Acknowledgements
All authors are participants of a collaborative research group dedicated to studying placebo and nocebo mechanisms across different physiological systems in health and disease. This work was supported by grants from the German Research Foundation (DFG) for the Research Unit FOR 1328 (BI 89/2-1; EN 50/30-1; RI 574/21-1; RI 574-22-1; SCHE 341/17-1), the Volkswagen Foundation Germany (P.E.: I/83 805; M.S.: I/83 806) and the German Federal Ministry of Education and Research (U.B.: 01GQ0808).
Author information
Paul Enck, Ulrike Bingel, Manfred Schedlowski and Winfried Rief: All authors contributed equally to this work.
Authors and Affiliations
Paul Enck is at the Department of Internal Medicine VI, University Hospital Tübingen, 72076 Tübingen, Germany.,
Ulrike Bingel is at the Department of Neurology, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.,
Ulrike Bingel
Manfred Schedlowski is at the Institute of Medical Psychology and Behavioural Immunobiology, University Hospital Essen, University of Duisburg-Essen, 45122 Essen, Germany.,
Manfred Schedlowski
Winfried Rief is at the Division of Clinical Psychology, University of Marburg, 35032 Marburg, Germany.,
Winfried Rief
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Paul Enck .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Related links
Further information.
Paul Enck's homepage
Ulrike Bingel's homepage
Manfred Schedlowski's homepage
Winfried Rief's homepage
A substance or treatment that mimics the side effects of the active compound under investigation and is thus, by definition, not an inert substance. In clinical trials, active placebos are administered to avoid un-blinding owing to different side-effect profiles of drugs and placebo treatments.
The ability of a clinical trial to differentiate between an effective treatment (for example, a drug) and a less effective or ineffective treatment (for example, placebo).
A comparative effectiveness research (CER) trial is performed to analyse the efficacy of a novel pharmacological agent or treatment in comparison with standard treatments or approved drugs. Patients are therefore randomly allocated to receive the treatment under investigation or one or more standard treatments.
A statement, developed by the World Medical Association (WMA), of ethical principles for medical research involving human participants, identifiable human material and data.
The extent to which individuals believe that they can control events that affect their personal health.
An experimental approach undertaken to separate the effects of the psychosocial context (placebo) from the pharmacodynamic effects of a drug under investigation. The pharmacological agent is administered either in an open condition (by a physician in a visible way) or in a hidden condition, in which the patient is unaware of the timing of the administration of the medication (for example, the drug is administered using computer-controlled infusion).
A method of application in which both the patients (or participants) and the investigators know which pharmacological agent or treatment is being administered. This design contrasts the single- or double-blind study designs.
(PROs). A method of measuring treatment efficacy via the states of symptom severity and health from the patient's perspective, instead of physician's reports or biomarkers of clinical outcome. PROs are typically analysed via questionnaires or interviews, providing insight into how patients perceive the impact of a treatment on their health and quality of life.
Latin term for “I shall please”. Used to indicate sham treatments or inert substances such as sugar pills or saline infusions.
Defined as any improvements in a symptom or physiological condition of individuals following a placebo treatment. There are different mechanisms underlying this phenomenon, including spontaneous remission, regression to the mean, natural course of a disease, biases and placebo responses.
The outcomes caused by a placebo manipulation. The placebo response reflects the neurobiological and psychophysiological response of an individual to an inert substance or sham treatment and is mediated by various factors that make up the treatment context. Importantly, placebo responses are not restricted to placebo treatments and can also modulate the outcome of any active treatment.
(RCTs). The most commonly used clinical trial design for testing the efficacy of a treatment within a patient population. Patients are randomly allocated to a treatment or placebo group. Patients and investigators are blinded to group allocation. The design aims to control for confounding factors such as suggestion, imagination and biases for the patient and investigator, as well as spontaneous fluctuation of diseases and symptoms.
A statistical phenomenon; individuals tend to have extreme values in symptom severity or physiological parameters when enrolled into a clinical trial. These values tend to be lower and closer to the average at subsequent assessments, because they are more likely to change in the direction of the mean score, instead of developing even more extreme scores. This phenomenon in part explains the improvement observed in placebo groups in clinical trials.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Enck, P., Bingel, U., Schedlowski, M. et al. The placebo response in medicine: minimize, maximize or personalize?. Nat Rev Drug Discov 12 , 191–204 (2013). https://doi.org/10.1038/nrd3923
Download citation
Published : 01 March 2013
Issue Date : March 2013
DOI : https://doi.org/10.1038/nrd3923
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Lumbale spinalkanalstenose - diagnostik und schmerztherapie.
- Heinrich Binsfeld
Schmerzmedizin (2024)
Placebo effects on nausea and motion sickness are resistant to experimentally-induced stress
- Carmen Jacob
- Elisabeth Olliges
- Karin Meissner
Scientific Reports (2023)
The impact of pharmaceutical form and simulated side effects in an open-label-placebo RCT for improving psychological distress in highly stressed students
- Alexander Winkler
- Alannah Hahn
- Christiane Hermann
Imaginary pills and open-label placebos can reduce test anxiety by means of placebo mechanisms
- Sarah Buergler
- Dilan Sezer
Neural underpinnings of open-label placebo effects in emotional distress
- Michael Schaefer
- Anja Kühnel
- Matti Gärtner
Neuropsychopharmacology (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Placebos and the Placebo Effect in Drug Trials
- First Online: 29 August 2019
Cite this chapter

- Paul Enck 13 &
- Sibylle Klosterhalfen 13
Part of the book series: Handbook of Experimental Pharmacology ((HEP,volume 260))
5447 Accesses
39 Citations
24 Altmetric
In this review, we explored different ways of controlling the placebo effects in clinical trials and described various factors that may increase/decrease the placebo effect in randomized placebo-controlled trials. These factors can be subdivided into four groups, and while not all factors are effective in every study and under all clinical conditions, they show on the whole that – even under the ideal condition of drug therapy, where blinded placebo provision is much easier and warranted than in, e.g., psychotherapy – many factors need to be controlled to ascertain that the goal of the clinical trials, fair assessment of superiority of the drug over placebo in placebo-controlled trials and fair assessment of non-inferiority of the drug compared to another drug in comparator trials, is reached. Ignorance towards the placebo effect, which was common in the past, is no longer acceptable; instead, it should be the goal of all therapeutic trials to minimize the placebo effect in clinical trials, while utilizing and maximizing it in clinical routine.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

What questions can a placebo answer?

Agid O, Siu CO, Potkin SG, Kapur S, Watsky E, Vanderburg D, Zipursky RB, Remington G (2013) Meta-regression analysis of placebo response in antipsychotic trials, 1970-2010. Am J Psychiatry 170(11):1335–1344
PubMed Google Scholar
Aslaksen PM, Myrbakk IN, Hoifodt RS, Flaten MA (2007) The effect of experimenter gender on autonomic and subjective responses to pain stimuli. Pain 129(3):260–268
Bakalos G, Zintzaras E (2018) Drug discontinuation in studies including a switch from an originator to a biosimilar monoclonal antibody: a systematic literature review. Clin Ther 41(1):155–173
Beecher HK (1955) The powerful placebo. J Am Med Assoc 159(17):1602–1606
CAS PubMed Google Scholar
Benedetti F (2014) Placebo effects. Oxford University Press, Oxford
Google Scholar
Benedetti F, Frisaldi E (2014) Creating placebo responders and nonresponders in the laboratory: boons and banes. Pain Manag 4(3):165–167
Benedetti F, Enck P, Frisaldi E, Schedlowski M (eds) (2014) Placebo, Handbook of experimental pharmacology, vol 225. Springer, Heidelberg
Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA (2009) Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 166(1):42–49
Burke MJ, Kaptchuk TJ, Pascual-Leone A (2018) Challenges of differential placebo effects in contemporary medicine: the example of brain stimulation. Ann Neurol 85(1):12–20
Chae Y, Lee YS, Enck P (2018) How placebo needles differ from placebo pills? Front Psych 9:243
Chey WD, Chey WY, Heath AT, Dukes GE, Carter EG, Northcutt A, Ameen VZ (2004) Long-term safety and efficacy of alosetron in women with severe diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 99(11):2195–2203
Colagiuri B, Schenk LA, Kessler MD, Dorsey SG, Colloca L (2015) The placebo effect: from concepts to genes. Neuroscience 307:171–190
CAS PubMed PubMed Central Google Scholar
Colloca L (2018a) Preface (part I). In: Neurobiology of the placebo effect part I. Academic Press, Cambridge, pp xv–xx
Colloca L (2018b) Preface (part II). In: Neurobiology of the placebo effect part II. Academic Press, Cambridge, pp xvii–xxiii
Colloca L, Benedetti F (2006) How prior experience shapes placebo analgesia. Pain 124(1–2):126–133
Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F (2010) How the number of learning trials affects placebo and nocebo responses. Pain 151(2):430–439
PubMed PubMed Central Google Scholar
Dal-Re R, Janiaud P, Ioannidis JPA (2018) Real-world evidence: how pragmatic are randomized controlled trials labeled as pragmatic? BMC Med 16(1):49
Darragh M, Booth RJ, Consedine NS (2014) Investigating the ‘placebo personality’ outside the pain paradigm. J Psychosom Res 76(5):414–421
De Allegri M, Pokhrel S, Becher H, Dong H, Mansmann U, Kouyate B, Kynast-Wolf G, Gbangou A, Sanon M, Bridges J, Sauerborn R (2008) Step-wedge cluster-randomised community-based trials: an application to the study of the impact of community health insurance. Health Res Policy Syst 6:10
de la Fuente-Fernandez R (2012) The powerful pre-treatment effect: placebo responses in restless legs syndrome trials. Eur J Neurol 19(10):1305–1310
Desai JR, Bowen EA, Danielson MM, Allam RR, Cantor MN (2013) Creation and implementation of a historical controls database from randomized clinical trials. J Am Med Inform Assoc 20(e1):e162–e168
Diener HC, Dowson AJ, Ferrari M, Nappi G, Tfelt-Hansen P (1999) Unbalanced randomization influences placebo response: scientific versus ethical issues around the use of placebo in migraine trials. Cephalalgia 19(8):699–700
Elsenbruch S, Enck P (2015) Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 12(8):472–485
Enck P, Klosterhalfen S (2019) Does sex/gender play a role in placebo and nocebo effects? Conflicting evidence from clinical trials and experimental studies. Front Neurosci 13:160
Enck P, Lackner JM (2019) Cognitive behavioural therapy for IBS: results or treatment as usual? Nat Rev Gastroenterol Hepatol. In press. https://doi.org/10.1038/s41575-019-0174-2
Enck P, Zipfel S (2019) Placebo effects in psychotherapy: a framework. Front Psych 10:456
Enck P, Klosterhalfen S, Kruis W (2005a) Determination of placebo effect in irritable bowel syndrome. Dtsch Med Wochenschr 130(34–35):1934–1937
Enck P, Klosterhalfen S, Kruis W (2005b) Factors affecting therapeutic placebo response rates in patients with irritable bowel syndrome. Nat Clin Pract Gastroenterol Hepatol 2(8):354–355
Enck P, Benedetti F, Schedlowski M (2008) New insights into the placebo and nocebo responses. Neuron 59(2):195–206
Enck P, Klosterhalfen S, Weimer K, Horing B, Zipfel S (2011a) The placebo response in clinical trials: more questions than answers. Philos Trans R Soc Lond Ser B Biol Sci 366(1572):1889–1895
Enck P, Klosterhalfen S, Zipfel S (2011b) Novel study designs to investigate the placebo response. BMC Med Res Methodol 11:90
Enck P, Grundy D, Klosterhalfen S (2012) A novel placebo-controlled clinical study design without ethical concerns – the free choice paradigm. Med Hypotheses 79(6):880–882
Enck P, Bingel U, Schedlowski M, Rief W (2013a) The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov 12(3):191–204
Enck P, Weimer K, Klosterhalfen S (2013b) Balanced placebo design, active placebos, and other design features for identifying, minimizing and characterizing the placebo response. In: Colloca L, Flaten MA, Meissner K (eds) Placebo and pain: from bench to bedside. Elsevier/Academic Press, Amsterdam, pp 159–173
Enck P, Horing B, Broelz E, Weimer K (2018) Knowledge gaps in placebo research: with special reference to neurobiology. Int Rev Neurobiol 139:85–106
Enck P, Weimer K, Colloca L, Doods S (eds) (2019) Placebo and nocebo effects in psychiatry and beyond. Front Psychiatr. Research Topic https://www.frontiersin.org/research-topics/7727/placebo-and-nocebo-effects-in-psychiatry-and-beyond#articles
Faasse K, Cundy T, Gamble G, Petrie KJ (2013) The effect of an apparent change to a branded or generic medication on drug effectiveness and side effects. Psychosom Med 75(1):90–96
Fava M, Evins AE, Dorer DJ, Schoenfeld DA (2003) The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom 72(3):115–127
FDA (2015) Mobile medical applications – guidance for industry and food and drug aministration staff. Federal Drug Administration, Silver Spring
FDA (2016) Non-inferiority clinical trials to establish effectiveness – guidance for industy. Federal Drug Administration, Silver Spring
Flight L, Julious SA (2016) Practical guide to sample size calculations: non-inferiority and equivalence trials. Pharm Stat 15(1):80–89
Ford AC, Moayyedi P (2010) Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther 32(2):144–158
Furukawa TA, Noma H, Caldwell DM, Honyashiki M, Shinohara K, Imai H, Chen P, Hunot V, Churchill R (2014) Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta-analysis. Acta Psychiatr Scand 130(3):181–192
Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR (2010) Dispositional optimism predicts placebo analgesia. J Pain 11(11):1165–1171
Gold SM, Enck P, Hasselmann H, Friede T, Hegerl U, Mohr DC, Otte C (2017) Control conditions for randomised trials of behavioural interventions in psychiatry: a decision framework. Lancet Psychiatry 4(9):725–732
Grelotti DJ, Kaptchuk TJ (2011) Placebo by proxy. BMJ 343:d4345
Grünbaum A (1986) The placebo concept in medicine and psychiatry. Psychol Med 16(1):19–38
Hall KT, Loscalzo J, Kaptchuk T (2018) Pharmacogenomics and the placebo response. ACS Chem Neurosci 9(4):633–635
Hesser H, Weise C, Rief W, Andersson G (2011) The effect of waiting: a meta-analysis of wait-list control groups in trials for tinnitus distress. J Psychosom Res 70(4):378–384
Horing B, Weimer K, Muth ER, Enck P (2014) Prediction of placebo responses: a systematic review of the literature. Front Psychol 5:1079
Horing B, Weimer K, Muth ER, Enck P (2015) Prediction of symptom change in placebo versus no-treatment group in experimentally induced motion sickness. Appl Psychophysiol Biofeedback 40(3):163–172
Horing B, Newsome ND, Enck P, Babu SV, Muth ER (2016) A virtual experimenter to increase standardization for the investigation of placebo effects. BMC Med Res Methodol 16(1):84
Irving G, Neves AL, Dambha-Miller H, Oishi A, Tagashira H, Verho A, Holden J (2017) International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open 7(10):e017902
Ivanova A, Tamura RN (2015) A two-way enriched clinical trial design: combining advantages of placebo lead-in and randomized withdrawal. Stat Methods Med Res 24(6):871–890
Jairath V, Zou G, Parker CE, Macdonald JK, Mosli MH, Khanna R et al (2016) Systematic review and meta-analysis: placebo rates in induction and maintenance trials of ulcerative colitis. J Crohns Colitis 10(5):607–618
Jensen KB, Petrovic P, Kerr CE, Kirsch I, Raicek J, Cheetham A, Spaeth R, Cook A, Gollub RL, Kong J, Kaptchuk TJ (2014) Sharing pain and relief: neural correlates of physicians during treatment of patients. Mol Psychiatry 19(3):392–398
Jensen JS, Bielefeldt AO, Hrobjartsson A (2017) Active placebo control groups of pharmacological interventions were rarely used but merited serious consideration: a methodological overview. J Clin Epidemiol 87:35–46
Jutte R (2013) The early history of the placebo. Complement Ther Med 21(2):94–97
Kaptchuk TJ (1998) Intentional ignorance: a history of blind assessment and placebo controls in medicine. Bull Hist Med 72(3):389–433
Kaptchuk TJ, Kelley JM, Deykin A, Wayne PM, Lasagna LC, Epstein IO, Kirsch I, Wechsler ME (2008) Do “placebo responders” exist? Contemp Clin Trials 29(4):587–595
Kelley JM, Lembo AJ, Ablon JS, Villanueva JJ, Conboy LA, Levy R, Marci CD, Kerr CE, Kirsch I, Jacobson EE, Riess H, Kaptchuk TJ (2009) Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom Med 71(7):789–797
Khan A, Redding N, Brown WA (2008) The persistence of the placebo response in antidepressant clinical trials. J Psychiatr Res 42(10):791–796
Khan S, Holbrook A, Shah BR (2018) Does Googling lead to statin intolerance? Int J Cardiol 262:25–27
Kirsch I (2000) Are drug and placebo effects in depression additive? Biol Psychiatry 47(8):733–735
Kirsch I (2014) The emperor’s new drugs: medication and placebo in the treatment of depression. Handb Exp Pharmacol 225:291–303
Kirsch I (2016) The placebo effect in the treatment of depression. Verhaltenstherapie 26:55–61
Klosterhalfen S, Kellermann S, Pan F, Stockhorst U, Hall G, Enck P (2005a) Effects of ethnicity and gender on motion sickness susceptibility. Aviat Space Environ Med 76(11):1051–1057
Klosterhalfen S, Kellermann S, Stockhorst U, Wolf J, Kirschbaum C, Hall G, Enck P (2005b) Latent inhibition of rotation chair-induced nausea in healthy male and female volunteers. Psychosom Med 67(2):335–340
Klosterhalfen S, Pan F, Kellermann S, Enck P (2006) Gender and race as determinants of nausea induced by circular vection. Gend Med 3(3):236–242
Kobak KA (2010) Inaccuracy in clinical trials: effects and methods to control inaccuracy. Curr Alzheimer Res 7(7):637–641
Kobak KA, Kane JM, Thase ME, Nierenberg AA (2007) Why do clinical trials fail? The problem of measurement error in clinical trials: time to test new paradigms? J Clin Psychopharmacol 27(1):1–5
Krogsboll LT, Hrobjartsson A, Gotzsche PC (2009) Spontaneous improvement in randomised clinical trials: meta-analysis of three-armed trials comparing no treatment, placebo and active intervention. BMC Med Res Methodol 9:1
Kronish IM, Hampsey M, Falzon L, Konrad B, Davidson KW (2018) Personalized (N-of-1) trials for depression: a systematic review. J Clin Psychopharmacol 38(3):218–225
Leal Rato M, Duarte GS, Ferreira AN, Alves M, Mainoli B, Teodoro T, Mestre TA, Costa J, Ferreira JJ (2019) Nocebo response in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. In press. https://doi.org/10.1016/j.parkreldis.2019.04.015
Legrain V, Iannetti GD, Plaghki L, Mouraux A (2011) The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol 93(1):111–124
Lipset CH (2014) Engage with research participants about social media. Nat Med 20(3):231
Liu Y, Rybin D, Heeren TC, Doros G (2019) Comparison of novel methods in two-way enriched clinical trial design. Stat Med. In press. https://doi.org/10.1002/sim.8288
Macaluso FS, Maida M, Ventimiglia M, Renna S, Cottone M, Orlando A (2018) Factors affecting clinical and endoscopic outcomes of placebo arm in trials of biologics and small molecule drugs in ulcerative colitis: a meta-analysis. Inflamm Bowel Dis 25(6):987–997
Maddocks M, Kerry R, Turner A, Howick J (2016) Problematic placebos in physical therapy trials. J Eval Clin Pract 22(4):598–602
Mahr A, Golmard C, Pham E, Lordache L, Deville L, Faure P (2017) Types, frequencies, and burden of nonspecific adverse events of drugs: analysis of randomized placebo-controlled clinical trials. Pharmacoepidemiol Drug Saf 26(7):731–741
Mallinckrodt CH, Zhang L, Prucka WR, Millen BA (2010) Signal detection and placebo response in schizophrenia: parallels with depression. Psychopharmacol Bull 43(1):53–72
Mathie RT, Ulbrich-Zurni S, Viksveen P, Roberts ER, Baitson ES, Legg LA, Davidson JRT (2018) Systematic review and meta-analysis of randomised, other-than-placebo controlled, trials of individualised homeopathic treatment. Homeopathy 107(4):229–243
May M (2019) Twenty-five ways clinical trials have changed in the last 25 years. Nat Med 25(1):2–5
Moncrieff J, Wessely S, Hardy R (2004) Active placebos versus antidepressants for depression. Cochrane Database Syst Rev 1:Cd003012
Moore RA, Wiffen PJ, Eccleston C, Derry S, Baron R, Bell RF, Furlan AD, Gilron I, Haroutounian S, Katz NP, Lipman AG, Morley S, Peloso PM, Quessy SN, Seers K, Strassels SA, Straube S (2015) Systematic review of enriched enrolment, randomised withdrawal trial designs in chronic pain: a new framework for design and reporting. Pain 156(8):1382–1395
Munkholm K, Paludan-Muller AS, Boesen K (2019) Considering the methodological limitations in the evidence base of antidepressants for depression: a reanalysis of a network meta-analysis. BMJ Open 9(6):e024886
Papakostas GI, Fava M (2009) Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol 19(1):34–40
Quessy SN, Rowbotham MC (2008) Placebo response in neuropathic pain trials. Pain 138(3):479–483
Rakel DP, Hoeft TJ, Barrett BP, Chewning BA, Craig BM, Niu M (2009) Practitioner empathy and the duration of the common cold. Fam Med 41(7):494–501
Rehn J, Schuster K (2017) Clinic design as placebo-using design to promote healing and support treatments. Behav Sci 7(4):E77
Relton C, Torgerson D, O’Cathain A, Nicholl J (2010) Rethinking pragmatic randomised controlled trials: introducing the “cohort multiple randomised controlled trial” design. BMJ 340:c1066
Rheker J, Rief W, Doering BK, Winkler A (2018) Assessment of adverse events in clinical drug trials: identifying amitriptyline’s placebo- and baseline-controlled side effects. Exp Clin Psychopharmacol 26(3):320–326
Rickels K, Lipman R, Raab E (1966) Previous medication, duration of illness and placebo response. J Nerv Ment Dis 142(6):548–554
Rief W, Nestoriuc Y, Weiss S, Welzel E, Barsky AJ, Hofmann SG (2009a) Meta-analysis of the placebo response in antidepressant trials. J Affect Disord 118(1–3):1–8
Rief W, Nestoriuc Y, von Lilienfeld-Toal A, Dogan I, Schreiber F, Hofmann SG, Barsky AJ, Avorn J (2009b) Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: a systematic review and meta-analysis. Drug Saf 32(11):1041–1056
Rutherford BR, Sneed JR, Roose SP (2009) Does study design influence outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychother Psychosom 78(3):172–181
Rutherford BR, Sneed JR, Tandler JM, Rindskopf D, Peterson BS, Roose SP (2011) Deconstructing pediatric depression trials: an analysis of the effects of expectancy and therapeutic contact. J Am Acad Child Adolesc Psychiatry 50(8):782–795
Schedlowski M, Enck P, Rief W, Bingel U (2015) Neuro-bio-behavioral mechanisms of placebo and Nocebo responses: implications for clinical trials and clinical practice. Pharmacol Rev 67(3):697–730
Schoenfeld DA, Finkelstein DM, Macklin E, Zach N, Ennist DL, Taylor AA, Atassi N (2019) Design and analysis of a clinical trial using previous trials as historical control. Clin Trials. https://doi.org/10.1177/1740774519858914
Shaffer JA, Kronish IM, Falzon L, Cheung YK, Davidson KW (2018) N-of-1 randomized intervention trials in Health Psychology: a systematic review and methodology critique. Ann Behav Med 52(9):731–742
Shah R, Ogden J (2006) ‘What’s in a face?’ The role of doctor ethnicity, age and gender in the formation of patients’ judgements: an experimental study. Patient Educ Couns 60(2):136–141
Shah E, Triantafyllou K, Hana AA, Pimentel M (2014) Adverse events appear to unblind clinical trials in irritable bowel syndrome. Neurogastroenterol Motil 26(4):482–488
Silverman RK, Ivanova A, Fine J (2018) Sequential parallel comparison design with binary and time-to-event outcomes. Stat Med 37(9):1454–1466
Spiller RC (1999) Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med 107(5a):91s–97s
Stein DJ, Baldwin DS, Dolberg OT, Despiegel N, Bandelow B (2006) Which factors predict placebo response in anxiety disorders and major depression? An analysis of placebo-controlled studies of escitalopram. J Clin Psychiatry 67(11):1741–1746
Stille G (1980) Preface. In: Handbook of experimental pharmacology, vol 55(I). Springer, Berlin, p V
Suchman AL, Ader R (1992) Classic conditioning and placebo effects in crossover studies. Clin Pharmacol Ther 52(4):372–377
Torous J, Firth J (2016) The digital placebo effect: mobile mental health meets clinical psychiatry. Lancet Psychiatry 3(2):100–102
Tsui M, Rehal S, Jairath V, Kahan BC (2019) Most non-inferiority trials were not designed to preserve active comparator treatment effects. J Clin Epidemiol 110:82–89
Vambheim SM, Flaten MA (2017) A systematic review of sex differences in the placebo and the nocebo effect. J Pain Res 10:1831–1839
Ventriglio A, Magnifico G, Borraccino L, Rinaldi A, Bellomo A (2018) Placebo and cultural responses. Nord J Psychiatry 72(supp 1):S33–S35
Wang RS, Hall KT, Giulianini F, Passow D, Kaptchuk TJ, Loscalzo J (2017) Network analysis of the genomic basis of the placebo effect. JCI Insight 2(11):93911
Wartolowska K, Judge A, Hopewell S, Collins GS, Dean BJ, Rombach I, Brindley D, Savulescu J, Beard DJ, Carr AJ (2014) Use of placebo controls in the evaluation of surgery: systematic review. BMJ 348:g3253
Waschbusch DA, Pelham WE Jr, Waxmonsky J, Johnston C (2009) Are there placebo effects in the medication treatment of children with attention-deficit hyperactivity disorder? J Dev Behav Pediatr 30(2):158–168
Wechsler ME, Kelley JM, Boyd IO, Dutile S, Marigowda G, Kirsch I, Israel E, Kaptchuk TJ (2011) Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med 365(2):119–126
Weimer K, Enck P (2014) Traditional and innovative experimental and clinical trial designs and their advantages and pitfalls. Handb Exp Pharmacol 225:237–272
Weimer K, Gulewitsch MD, Schlarb AA, Schwille-Kiuntke J, Klosterhalfen S, Enck P (2013) Placebo effects in children: a review. Pediatr Res 74(1):96–102
Weimer K, Colloca L, Enck P (2015a) Age and sex as moderators of the placebo response – an evaluation of systematic reviews and meta-analyses across medicine. Gerontology 61(2):97–108
Weimer K, Colloca L, Enck P (2015b) Placebo effects in psychiatry: mediators and moderators. Lancet Psychiatry 2(3):246–257
Whalley B, Hyland ME, Kirsch I (2008) Consistency of the placebo effect. J Psychosom Res 64(5):537–541
White P, Lewith G, Hopwood V, Prescott P (2003) The placebo needle, is it a valid and convincing placebo for use in acupuncture trials? A randomised, single-blind, cross-over pilot trial. Pain 106(3):401–409
Wolf S (1959) The pharmacology of placebos. Pharmacol Rev 11:689–704
Zelen M (1979) A new design for randomized clinical trials. N Engl J Med 300(22):1242–1245
Zhu Z, Zhang L, Jiang J, Li W, Cao X, Zhou Z, Zhang T, Li C (2014) Comparison of psychological placebo and waiting list control conditions in the assessment of cognitive behavioral therapy for the treatment of generalized anxiety disorder: a meta-analysis. Shanghai Arch Psychiatry 26(6):319–331
Download references
Acknowledgments
The assistence of Astrid Bahun from Bahun + Design for the artwork is gratefully acknowledged.
Author information
Authors and affiliations.
Department of Internal Medicine VI: Psychosomatic Medicine and Psychotherapy , University Hospital Tübingen, Tübingen, Germany
Paul Enck & Sibylle Klosterhalfen
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Paul Enck .
Editor information
Editors and affiliations.
Center for Substance Abuse Research, Lewis Katz School of Medicine at Temple University, Philadelphia, PA, USA
James E. Barrett
Sackler Institute of Pulmonary Pharmacology, Institute of Pharmaceutical Science, King’s College London, London, UK
Clive P. Page
Department of Pharmacology, Johannes Gutenberg University, Mainz, Rheinland-Pfalz, Germany
Martin C. Michel
Rights and permissions
Reprints and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Enck, P., Klosterhalfen, S. (2019). Placebos and the Placebo Effect in Drug Trials. In: Barrett, J., Page, C., Michel, M. (eds) Concepts and Principles of Pharmacology. Handbook of Experimental Pharmacology, vol 260. Springer, Cham. https://doi.org/10.1007/164_2019_269
Download citation
DOI : https://doi.org/10.1007/164_2019_269
Published : 29 August 2019
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-35361-2
Online ISBN : 978-3-030-35362-9
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
VIDEO
COMMENTS
Placebo-controlled studies are a way of testing a medical therapy in which, in addition to a group of subjects that receives the treatment to be evaluated, a separate control group receives a sham "placebo" treatment which is specifically designed to have no real effect.
Along with its importance in clinical trial design, the placebo response represents an active pain-modulatory process that engages pain-inhibitory systems via multiple mechanisms. As such, the placebo response provides a valuable opportunity for investigating endogenous pain-control systems.
The randomized clinical trial was a major methodological breakthrough in medicine and the best evidence for new treatment came from randomized placebo-controlled (RCT) double-blind studies. It was noticed that patients improved, sometimes dramatically, in placebo control arms.
Randomized, placebo controlled trials (PCTs) are widely considered to be the most rigorous method of evaluating the efficacy of treatment or prevention interventions. To be ethical, clinical research requires balancing rigorous science with the protection of human subjects.
The use of a placebo control intervention to induce and maintain blinding reduces the risk of bias (Schulz 2002; Hróbjartsson 2011a). Placebos enable blinding by mimicking the experience of receiving an experimental intervention without containing any supposed therapeutic components.
Investigating the size and mechanisms of the placebo response in different medical conditions has relied on experimental procedures that simulate the double-blind randomized placebo-controlled design of clinical trials.
There are three major areas in which placebo interventions have an important role: (i) as control interventions in experimental studies to determine specific effects and to reduce bias by enabling blinding; (ii) as experimental interventions in placebo research to study placebo effects; (iii) as a tool in clinical practice.
For many years, randomized double-blind placebo-controlled trials (RCTs) have been conducted to disentangle the specific effects of a therapeutic intervention — such as the administration...
A trial that uses a placebo is described as a “placebo-controlled trial.” In this type of study, the test group receives the experimental treatment, and the control group receives the placebo. Placebos are not used if an effective treatment is already available or if you would be put at risk by not having effective therapy.
Placebo effect. 1 Introduction. In both traditional and modern pharmacology, placebos are understood as tools, as research vehicles with which the true efficacy and mechanism of action of “real” drugs can be elucidated.