- Structure of Atom
- Oil Drop Experiment

Milliken's Oil Drop Experiment
The Millikens Oil Drop Experiment was an experiment performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the charge of an electron. This experiment proved to be very crucial in the physics community.
Millikens Oil Drop Experiment Definition
In the experiment, Milliken allowed charged tiny oil droplets to pass through a hole into an electric field. By varying the strength of the electric field the charge over an oil droplet was calculated, which always came as an integral value of ‘e.’
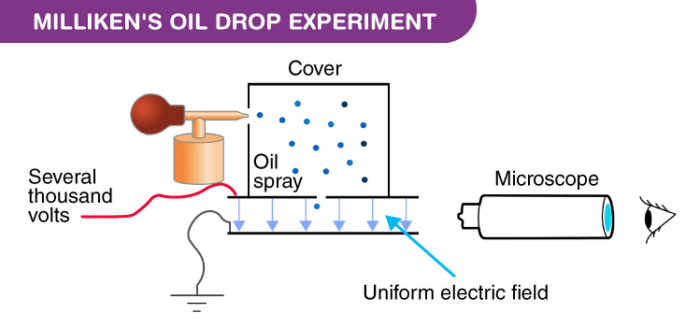
Apparatus of the Milliken’s Oil Drop Experiment
The apparatus for the experiment was constructed by Milliken and Fletcher. It incorporated two metal plates held at a distance by an insulated rod. There were four holes in the plate, out of which three were there to allow light to pass through them and one was there to allow viewing through the microscope.
Ordinary oil wasn’t used for the experiment as it would evaporate by the heat of the light and so could cause an error in the Millikens Oil Drop Experiment. So, the oil that is generally used in a vacuum apparatus which is of low vapour pressure was used.
Milliken’s Oil Drop Experiment Procedure
- Oil is passed through the atomizer from where it came in the form of tiny droplets. They pass the droplets through the holes present in the upper plate of the apparatus.
- The downward motions of droplets are observed through a microscope and the mass of oil droplets, then measure their terminal velocity.
- The air inside the chamber is ionized by passing a beam of X-rays through it. The electrical charge on these oil droplets is acquired by collisions with gaseous ions produced by ionization of air.
- The electric field is set up between the two plates and so the motion of charged oil droplets can be affected by the electric field.
- Gravity attracts the oil in a downward direction and the electric field pushes the charge upward. The strength of the electric field is regulated so that the oil droplet reaches an equilibrium position with gravity.
- The charge over the droplet is calculated at equilibrium, which is dependent on the strength of the electric field and mass of droplet.
Milliken’s Oil Drop Experiment Calculation
F up = F down
F up = Q . E
F down = m.g
Q is an electron’s charge, E is the electric field, m is the droplet’s mass, and g is gravity.
One can see how an electron charge is measured by Millikan. Millikan found that all drops had charges that were 1.6x 10 -19 C multiples.
Milliken’s Oil Drop Experiment Conclusion
The charge over any oil droplet is always an integral value of e (1.6 x 10 -19 ). Hence, the conclusion of Millikens Oil Drop Experiment is that the charge is said to be quantized, i.e. the charge on any particle will always be an integral multiple of e.
Frequently Asked Questions – FAQs
What did millikan’s oil drop experiment measure.
Millikan oil-drop test, the first simple and persuasive electrical charge calculation of a single electron. It was first conducted by the American physicist Robert A. in 1909. He discovered that all the drops had charges that were simple multiples of a single integer, the electron’s fundamental charge.
What is the importance of Millikan’s oil drop experiment?
The experiment with Millikan is important since it defined the charge on an electron. Millikan used a very basic, very simple system in which the behaviour of gravitational, electrical, and (air) drag forces were controlled.
What did Millikan conclude after performing his oil drop experiment?
An integral multiple of the charge on an electron is the charge on every oil decrease. About an electric force. In a relatively small amount, the charge and mass of the atom must be condensed.

Why charges are quantized?
Charges are quantized since every object’s charge (ion, atom, etc.) Charge quantization, therefore, implies that no random values can be taken from the charge, but only values that are integral multiples of the fundamental charge (proton / electron charge).
Can charge be created or destroyed?
The Charge Conservation Law does not suggest that it is difficult to generate or remove electrical charges. It also means that any time a negative electrical charge is produced, it is important to produce an equal amount of positive electrical charge at the same time so that a system’s overall charge does not shift.
For more information about quantum physics , download BYJU’S-The learning app to play store and app store.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
Description This simulation is a simplified version of an experiment done by Robert Milliken in the early 1900s. Hoping to learn more about charge, Milliken sprayed slightly ionized oil droplets into an electric field and made observations of the droplets. When the voltage is zero and the run button is pressed, the drop will fall due to the force of gravity. It will reach a terminal velocity (v t ) as it falls. Pause the simulation while you record the terminal velocity. This terminal velocity can be used to determine the mass of the drop. Use the equation: mass = kv t 2 to determine the mass of the particle. The value of k in this simulation is 4.086 x 10 -17 kg s 2 /m 2 . Once the terminal velocity is recorded and the mass calculated, with the simulation still paused increase the voltage between the plates until the two force vectors are approximately equal length. This will produce an upward field and an upward force on the positive droplets. If the upward force of the electric field is equal to the downward force of gravity, and the drag force is zero, the particle will not accelerate. To be sure that the lack of acceleration is not related to drag forces, the velocity must also be zero as well as the acceleration in order to be sure that the two forces are balanced. Increase and decrease the voltage (use the left/right arrow keys) until both the acceleration and velocity are at zero. The velocity may not stay at exactly zero, but find the voltage that has the velocity changing most slowly as it passes v = 0. Use the methods discussed above to ultimately determine the charge on ten (or more) different oil-drops. Use V = Ed to calculate the field strength (d = 5 cm = 0.05 m). Use Eq = mg when the velocity is zero to determine the charge q on the droplet. Record all your data in a table or spreadsheet. After you get each q, create a new particle and start again. When you have the table filled in, look at the various values for q. Is there any pattern to them, or are they seemingly random? Can you draw any conclusions from the Q measurements?
Talk to our experts
1800-120-456-456
- Millikan's Oil Drop Experiment

Introduction
The oil drop experiment was performed in 1909 by Robert A. Millikan and Harvey Fletcher to measure the elementary electric charge (it means the electron's charge). This experiment took place in the Ryerson Physical Laboratory, which is present at the University of Chicago. Also, this experiment has proved to be very crucial in physics.
Before this experiment, the existence of subatomic particles was not accepted universally. Millikan's apparatus has an electric field created between a parallel pair of metal plates held apart by an insulating material. The oil droplets, which are electrically charged, enter the electrical field and are balanced between two plates by altering the field. When the charged drops fell at a constant rate, the gravitational forces and electric forces on it were equal.
Principles of Millikan's Experiment
The Millikan experiment is complicated and fiddly while performing in school. It is more likely that we will use a simulation or a film clip of the experiment to show its principles to the students. Few of such principles are,
An oil drop can fall under its own weight. If a charge is given to the drop, it can be suspended by using an electric field. At this point, the electrostatic force balances the weight of every drop. Then the size of the electrostatic force depends entirely on the drop. So Millikan should have figured out the charge as soon as he knew the weight.
Millikan allowed the drop to fall through the air to find the weight of the drop. It reaches its terminal velocity quickly. At this point, the weight is balanced by the viscous drag of the air. Drag can be calculated from the Stokes' Law, which allowed Millikan to determine the weight.
Millikan repeated the same experiment thoroughly for over 150 oil drops and selected 58 of Millikan oil drop experiment results and got to find the highest common factor. It means the single unit of charge that could be multiplied up to give the charge he measured on all of his oil drops.
Oil Drop Experiment
Millikan allowed charged small oil droplets to travel through a hole into an electric field in the experiment. With the electric field's varying strength, the charge over an oil droplet is calculated, and it always comes as a fundamental value of 'e.'
(Image will be uploaded soon)
Millikan and Fletcher designed the experiment apparatus. It included two metal plates held at a distance by an insulated rod. There were four holes in the plate, three of which were there to allow light to pass through, and one was there to allow viewing through the microscope.
They did not use ordinary oil for this experiment, as it would evaporate by the heat of the light, and could cause an error in the Millikan Oil Drop Experiment. The oil, which is usually used in a vacuum apparatus with low vapour pressure, was also used.
Oil passes through the atomizer, from where it came in tiny droplets form. The same droplets pass through the holes in the upper plate of the apparatus.
The droplet's downward movements are observed through the microscope and the mass of the oil droplets, and then their terminal velocity is measured.
The air present inside the chamber is ionised by passing through the X-ray beam. Collisions obtain the electrical charge on these oil droplets with gaseous ions produced by the ionisation of air.
Then, the electric field is set up between the two plates so that the motion of the charged oil droplets can be affected by the same electric field.
Now, gravity attracts the oil in a downward direction, and the electric field pushes the charge upwards. Also, the electric field strength is regulated so that all the oil droplets reach an equilibrium position with gravity.
The charge on the droplet is calculated at equilibrium, which depends on the mass of the droplet and strength of the electric field.
Millikan Oil Drop Experiment Calculations
The experiment initially allows the oil drops to fall between the plates in the absence of the electric field. They accelerate first due to gravity, but gradually the oil droplets slow down because of air resistance.
The Millikan oil drop experiment formula can be given as below.
F up = Q ⋅ E F down = m
Where Q is an electron’s charge, m is the droplet’s mass, E is the electric field, and g is gravity.
Q ⋅ E = m ⋅ g
By this, one can identify how an electron charge is measured by Millikan. Millikan also found that all the drops had charges, which were 1.6x 10 -19 C multiples.
Importance of Millikan's Oil Drop Experiment
Millikan's experiment is quite essential because it establishes the charge on an electron.
Millikan used a simple apparatus in which he balanced the actions of electric, gravitational, and air drag forces.
Using the apparatus, he was able to calculate the charge on an electron as 1.60 × 10 -19 C.
The charge for any oil droplet is always an integral value of e (1.6 x 10 -19 ). Thus, Millikan's Oil Drop Experiment concludes that the charge is said to be quantized, which means that the charge on any particle will be an integral multiple of e always.
Millikan discovered the charge on a single electron using a uniform electric field between the oil drops and two parallel charged plates.
FAQs on Millikan's Oil Drop Experiment
1. What is Millikan’s Oil Drop experiment?
In 1909, Robert Millikan and Harvey Fletcher conducted the canvas drop trial to determine the charge of an electron. They suspended bitsy charged driblets of canvas between two essence electrodes by balancing downcast gravitational force with upward drag and electric forces. The viscosity of the canvas was known, so Millikan and Fletcher could determine the driblets’ millions from their observed diameters (since from the diameters they could calculate the volume and therefore, the mass). Using the known field and therefore the values of graveness and mass, Millikan and Fletcher determined the charge on canvas driblets in mechanical equilibrium. By repeating the trial, they verified that the charges were all multiples of some abecedarian value. They calculated this value to be1.5924 × 10 −19 Coulombs (C), which is within 1 of the presently accepted value of1.602176487 × 10 −19 C. They proposed that this was the charge of one electron.
2. How did the process work?
The outfit incorporated a brace of essence plates and a specific type of canvas. Millikan and Fletcher discovered it had been stylish to use a canvas with a particularly low vapor pressure, similar together designed to be used during a vacuum outfit. Ordinary canvas would dematerialize under the heat of the light source, causing the mass of the canvas to drop to change over the course of the trial.
By applying an implicit difference across a resemblant brace of vertical essence plates, an invariant electric field was created in the space between them. A ring of separating material was used to hold the plates piecemeal. Four holes were dug into the ring — three for illumination by a bright light and another to permit viewing through a microscope. A fine mist of canvas driblets was scattered into a chamber above the plates. The canvas drops came electrically charged through disunion with the snoot as they were scattered. Alternatively, the charge could be convinced by including an ionizing radiation source ( similar to an X-ray tube).
3. Describe the Millikan’s Oil Drop experiment procedure?
Canvas is passed through the atomizer from where it came in the form of bitsy driblets. They pass the driblets through the holes present in the upper plate of the outfit.
The downcast movements of driblets are observed through a microscope and the mass of canvas driblets also measure their terminal haste.
The air inside the chamber is ionised by passing a ray of X-rays through it. The electrical charge on these canvas driblets is acquired by collisions with gassy ions produced by the ionisation of air.
The electric field is set up between the two plates and so the stir of charged canvas driblets can be affected by the electric field.
Graveness attracts the canvas in a downcast direction and the electric field pushes the charge overhead. The strength of the electric field is regulated so that the canvas drop reaches an equilibrium position with graveness.
The charge over the drop is calculated at equilibrium, which depends on the strength of the electrical field and the mass of the drop.
4. Explain Millikan’s Oil Drop experiment in detail?
Millikan’s original trial or any modified interpretation, similar to the following, is called the canvas-drop trial. An unrestricted chamber with transparent sides is fitted with two resemblant essence plates, which acquire a positive or negative charge when an electric current is applied. At the launch of the trial, an atomizer sprays a fine mist of canvas driblets into the upper portion of the chamber. Under the influence of gravity and air resistance, some of the canvas driblets fall through a small hole cut in the top essence plate. When the space between the essence plates is ionized by radiation (e.g., X-rays), electrons from the air attach themselves to the falling canvas driblets, causing them to acquire a negative charge.
A light source, set at right angles to a viewing microscope, illuminates the canvas driblets and makes them appear as bright stars while they fall. The mass of a single charged drop can be calculated by observing how presto it falls. By confirming the implicit difference, or voltage, between the essence plates, the speed of the drop’s stir can be increased or dropped; when the quantum of upward electric force equals the given downcast gravitational force, the charged drop remains stationary. The quantum of voltage demanded to suspend a drop is used along with its mass to determine the overall electric charge on the drop.
Through the repeated operation of this system, the values of the electric charge on individual canvas drops are always whole- number multiples of the smallest value — that value being the abecedarian electric charge itself (about1.602 × 10 −19 coulomb). From the time of Millikan’s original trial, this system offered satisfying evidence that electric charge exists in introductory natural units. All posterior distinct styles of measuring the introductory unit of electric charge point to its having the same abecedarian value.
5. How does Millikan’s Oil Drop experiment work?
Simplified scheme of Millikan’s canvas-drop trial This outfit has a resemblant brace of vertical essence plates. An invariant electric field is created between them. The ring has three holes for illumination and one for viewing through a microscope. A specific type of canvas is scattered into the chamber, where drops come electrically charged. The driblets enter the space between the plates and can be controlled by changing the voltage across the plates.
The driblets entered the space between the plates and, because they were charged, they could be controlled by changing the voltage across the plates. Originally, the canvas drops were allowed to fall between the plates with the electric field turned off. The snappily reached terminal haste due to disunion with the air in the chamber. The field was turned on and, if it was large enough, some of the drops (the charged bones) would start to rise. This is because the overhead electric force, FE, is lesser for them than the down gravitational force,g. (A charged rubber rod can pick up bits of paper in the same way.) A likely-looking drop was named and kept in the middle of the field of view by alternatively switching off the voltage until all the other drops fell. The trial was continued with this single drop. Millikan’s canvas drop trial measured the charge of an electron. Before this trial, the actuality of subatomic patches wasn't widely accepted.
Millikan’s outfit contained an electric field created between a resemblant brace of essence plates, which were held piecemeal by separating material. Electrically charged canvas driblets entered the electric field and were balanced between two plates by altering the field.
6. Why was the Negative Plate Earthed in Millikan's Oil Drop Experiment?
There are three possible reasonable ways to clear it.
The first reason is safety. Grounding ("earthing," in this context), the equipment is so important, particularly the time when you are working with high voltages. The same would be applied to protecting the equipment and for personal safety as well.
The second reason would be to establish a good stable reference point for the voltage measurement. A massive and solidly connected grounding cable would perform that job in a better way.
Finally, from an electrical standpoint, the two plates used in Millikan's experiment form a capacitor. On the other side, this capacitor is being charged to a very high voltage. In such cases, it is suggested to have a discharge path on one of the terminals or plates in order to avoid damage to either humans or equipment as well. Therefore, the negative plate is earthed.
7. Why do we Use Oil Instead of Other Liquids in the Millikan Oil-drop Experiment?
Oil is one of the best liquids for Millikan's oil drop experiment. It retains its mass over a while and exposes to higher temperatures. Also, we employ an atomizer for ultra-fine droplets. So less dense liquids like water and oils are preferred over water because water cannot survive at such higher temperatures.
The atomizer employment is also an important reason behind using oil for this experiment. Moreover, it should be noted that oil would retain the exact volume/mass/weight. This would enable an exact measurement of the charge. Other liquids would separate or dissipate or even evaporate.


IMAGES
COMMENTS
Millikan's oil-drop experiment was performed by Robert Millikan and Harvey Fletcher in 1909. It determined a precise value for the electric charge of the electron, e. The electron's charge is the fundamental unit of electric charge because all electric charges are made up of groups (or the absence of groups) of electrons.
One can see how an electron charge is measured by Millikan. Millikan found that all drops had charges that were 1.6x 10-19 C multiples. Milliken’s Oil Drop Experiment Conclusion. The charge over any oil droplet is always an integral value of e (1.6 x 10-19). Hence, the conclusion of Millikens Oil Drop Experiment is that the charge is said to ...
Route of charge calculation. Origin projects. Data analysis. Project: Millikan1.opj Locations: \\Phyaplportal\PHYCS401\Common\Origin templates\Oil drop experiment \\Phyaplportal\PHYCS401\Students\Oil drop experiment Please make a copy (not move!) of Millikan1.opj in your personal folder and start to work with your personal copy of the project 9 ...
this experiment will be in the range of 0.01 to 0.001 cm/s, a correction factor must be included in the expression for en. This factor is: 3/ 2 1 / 1 +b pa (7) where b is a constant, p is the atmospheric pressure, and a is the radius of the drop as calculated from equation (5). Using the correction factor, the charge on the droplet is
and used by R.A. Millikan to show that electric charge exists as integral multiples of the elementary charge of a single electron. This experiment first described by [Millikan, 1913] is based on the fact that different forces act on an electrically charged oil drop moving in the homogeneous electric field of a plate capacitor (Figure 1).
of electron and Avogadro’s number. It won Millikan the Nobel Prize in the year 1923. This experiment depends on the ability to control, measure and balance very small force of the order 10-14 N. The set-up consists of two horizontal parallel plates separated by about 5mm.
This simulation is a simplified version of an experiment done by Robert Milliken in the early 1900s. Hoping to learn more about charge, Milliken sprayed slightly ionized oil droplets into an electric field and made observations of the droplets. When the voltage is zero and the run button is pressed, the drop will fall due to the force of gravity.
1. Oil drop experiment. Robert A. Millikan.. (1909). e=1.5924(17)×10−19C 2. Shot noise experiment. First proposed by Walter H. Schottky 3. In terms of the Avogadro constant and Faraday constant 𝒆= 𝑵𝑨; F- Faraday constant, N A - Avagadro constant. Best uncertainty ~1.6 ppm. 4. From Josephson ( = 𝒆 )and von Klitzing 𝑹 = 𝒆
Millikan Oil Drop Experiment Calculations. The experiment initially allows the oil drops to fall between the plates in the absence of the electric field. They accelerate first due to gravity, but gradually the oil droplets slow down because of air resistance. The Millikan oil drop experiment formula can be given as below. F up = Q ⋅ E F down = m
Millikan's Oil Drop Experiment At the start of the 20th century, scientists discovered the existence of the electron. They knew it possessed a mass and an electrical charge, and they had determined the charge-to-mass ratio, e/m. In 1909, Robert Millikan and Harvey Fletcher developed an experiment to determine the fundamental charge of the electron.