You are using an outdated browser. Please upgrade your browser to improve your experience.
OR, Choose a Category

Kool Aid Tie Dye – Chemical Bond

When the atoms in different kinds of molecules come together they can form a chemical bond . This happens when some of the electrons from each kind of atom have an attraction to each other so they stick together. In this experiment you will be able to see a chemical bond . Dye made from kool aid and vinegar will make a bond, or “stick” to the fabric of a cotton t-shirt – kool aid tie dye!
Some chemical bonds are strong and the two substances really stick to each other. Some chemical bonds are weak. The chemical bond between kool-aid/vinegar and the t-shirt is weak. The vinegar added to the kool-aid is called a mordant . A mordant is a substance that helps dye stick to fabric.
What You Need to Make Kool Aid Tie Dye

- T-shirt/Sock/Towel – Anything Made From Cotton
- Kool Aid Packets
- Several Bowls
- Measuring Cups
- Rubber Bands
The kool-aid/vinegar dye will make a weak chemical bond so your shirt will fade over time. The chemical bond in a permanent dye is strong – shirts dyed with this kind of dye stay bright for a long time. After you practice with kool-aid, THEN try a more permanent dye.
NOTE : Even though the kool-aid/vinegar dye is weak…you should still do this OUTSIDE! The kool-aid/vinegar dye will stay on your fingers and especially your fingernails for a day or so unless you wash them really good. (So…it would also stay for awhile on your clothes or the carpet in your house!) My dog licked the bowl of blue kool-aid/vinegar dye and it turned her tongue blue. She also splashed some on her foot. The next day her tongue was not blue anymore put the fur on her paw was!
Science Experiment Idea : Try dying three identical shirts with kool aid using different amounts of vinegar. Which mixture made the darkest color? Which mixture lasted the longest? To investigate chemical reactions further – try some more experiments at home!
Websites, Activities & Printables
- Steve Spangler: The Science of Tie Dye
- Scientific American: Making Tie Dye T-shirts
- Khan Academy: Chemical Bonds
- Kool Aid: Learn How to Dye Easter Eggs, Yarn, and Hair!

You can also ask a math and science expert for homework help by calling the A s k Rose Homework Hotline . They provide FREE math and science homework help to Indiana students in grades 6-12.
e-Books and Audiobooks
Use your indyPL Library Card to check out books about Science Experiments at any of our locations , or check out science experiment e-books and audiobooks from OverDrive Kids right to your device! If you have never used OverDrive before, you can learn how to use e-books and learn how to use audiobooks .
Need more help? Ask a Library staff member at any of our locations or call, text or email Ask-a-Librarian . Additionally, the Tinker Station helpline at (317) 275-4500 is also available. It is staffed by device experts who can answer questions about how to read, watch and listen on a PC, tablet or phone.
Chemistry for Kids: Projects to Makes Things Sizzle, Pop, and Explode!
Chemistry is nature’s magic. With it you can learn to do amazing things, like make erupting volcanoes and and fizzy exploding ziplocs. These books will show you how to do these things and also explain the science behind why these things are happening. You can explore chemical reactions by experimenting with things you find around the house in your kitchen, bathroom or garage.
View more…
- Tags Homework Help , Science Experiments

68 Best Chemistry Experiments: Learn About Chemical Reactions
Whether you’re a student eager to explore the wonders of chemical reactions or a teacher seeking to inspire and engage your students, we’ve compiled a curated list of the top 68 chemistry experiments so you can learn about chemical reactions.
While the theories and laws governing chemistry can sometimes feel abstract, experiments bridge the gap between these concepts and their tangible manifestations. These experiments provide hands-on experiences illuminating the intricacies of chemical reactions, molecular structures, and elemental properties.
1. Covalent Bonds

By engaging in activities that demonstrate the formation and properties of covalent bonds, students can grasp the significance of these bonds in holding atoms together and shaping the world around us.
Learn more: Covalent Bonds
2. Sulfuric Acid and Sugar Demonstration
Through this experiment, students can develop a deeper understanding of chemical properties, appreciate the power of chemical reactions, and ignite their passion for scientific exploration.
3. Make Hot Ice at Home
Making hot ice at home is a fascinating chemistry experiment that allows students to witness the captivating transformation of a liquid into a solid with a surprising twist.
4. Make a Bouncing Polymer Ball

This hands-on activity not only allows students to explore the fascinating properties of polymers but also encourages experimentation and creativity.
Learn more: Thought Co
5. Diffusion Watercolor Art

This experiment offers a wonderful opportunity for students to explore the properties of pigments, observe how they interact with water, and discover the mesmerizing patterns and textures that emerge.
Learn more: Diffusion Watercolor Art
6. Exploding Baggie

The exploding baggie experiment is a captivating and dynamic demonstration that students should engage in with caution and under the supervision of a qualified instructor.
Learn more: Exploding Baggie
7. Color Changing Chemistry Clock

This experiment not only engages students in the world of chemical kinetics but also introduces them to the concept of a chemical clock, where the color change acts as a timekeeping mechanism.
Learn more: Color Changing Chemistry Clock
8. Pipe Cleaner Crystal Trees

By adjusting the concentration of the Borax solution or experimenting with different pipe cleaner arrangements, students can customize their crystal trees and observe how it affects the growth patterns.
Learn more: Pipe Cleaner Crystal Trees
9. How To Make Ice Sculptures

Through this experiment, students gain a deeper understanding of the physical and chemical changes that occur when water freezes and melts.
Learn more: Ice Sculpture
10. How to Make Paper

Through this hands-on activity, students gain a deeper understanding of the properties of cellulose fibers and the transformative power of chemical reactions.
Learn more: How to Make Paper
11. Color Changing Chemistry
Color changing chemistry is an enchanting experiment that offers a captivating blend of science and art. Students should embark on this colorful journey to witness the mesmerizing transformations of chemicals and explore the principles of chemical reactions.
12. Gassy Banana
The gassy banana experiment is a fun and interactive way for students to explore the principles of chemical reactions and gas production.
Learn more: Gassy Banana
13. Gingerbread Man Chemistry Experiment

This hands-on activity not only introduces students to the concepts of chemical leavening and heat-induced reactions but also allows for creativity in decorating and personalizing their gingerbread creations.
Learn more: Gingerbread Man Chemistry Experiment
14. Make Amortentia Potion

While the love potion is fictional, this activity offers a chance to explore the art of potion-making and the chemistry behind it.
Learn more: How to Make Amortentia Potion
15. Strawberry DNA Extraction
This hands-on experiment offers a unique opportunity to observe DNA, the building blocks of life, up close and learn about its structure and properties.
16. Melting Snowman

The melting snowman experiment is a fun and whimsical activity that allows students to explore the principles of heat transfer and phase changes.
Learn more: Melting Snowman
17. Acid Base Cabbage Juice

The acid-base cabbage juice experiment is an engaging and colorful activity that allows students to explore the pH scale and the properties of acids and bases.
By extracting the purple pigment from red cabbage leaves and creating cabbage juice, students can use this natural indicator to identify and differentiate between acidic and basic substances.
Learn more: Acid Base Cabbage Juice
18. Magic Milk

The magic milk experiment is a mesmerizing and educational activity that allows students to explore the concepts of surface tension and chemical reactions.
By adding drops of different food colors to a dish of milk and then introducing a small amount of dish soap, students can witness a captivating display of swirling colors and patterns.
Learn more: Magic Milk
19. Melting Ice with Salt and Water

Through this hands-on activity, students can gain a deeper understanding of the science behind de-icing and how different substances can influence the physical properties of water.
Learn more: Melting Ice with Salt and Water
20. Barking Dog Chemistry Demonstration

The barking dog chemistry demonstration is an exciting and visually captivating experiment that showcases the principles of combustion and gas production.
21. How to Make Egg Geodes

Making egg geodes is a fascinating and creative chemistry experiment that students should try. By using common materials like eggshells, salt, and food coloring, students can create their own beautiful geode-like crystals.
Learn more: How to Make Egg Geodes
22. Make Sherbet

This experiment not only engages the taste buds but also introduces concepts of acidity, solubility, and the chemical reactions that occur when the sherbet comes into contact with moisture.
Learn more: Make Sherbet
23. Hatch a Baking Soda Dinosaur Egg

As the baking soda dries and hardens around the toy, it forms a “shell” resembling a dinosaur egg. To hatch the egg, students can pour vinegar onto the shell, causing a chemical reaction that produces carbon dioxide gas.
Learn more: Steam Powered Family
24. Chromatography Flowers

By analyzing the resulting patterns, students can gain insights into the different pigments present in flowers and the science behind their colors.
Learn more: Chromatography Flowers
25. Turn Juice Into Solid

Turning juice into a solid through gelification is an engaging and educational chemistry experiment that students should try. By exploring the transformation of a liquid into a solid, students can gain insights of chemical reactions and molecular interactions.
Learn more: Turn Juice into Solid
26. Bouncy Balls
Making bouncy balls allows students to explore the fascinating properties of polymers, such as their ability to stretch and rebound.
27. Make a Lemon Battery
Creating a lemon battery is a captivating and hands-on experiment that allows students to explore the fundamentals of electricity and chemical reactions.
28. Mentos and Soda Project
The Mentos and soda project is a thrilling and explosive experiment that students should try. By dropping Mentos candies into a bottle of carbonated soda, an exciting eruption occurs.
29. Alkali Metal in Water
The reaction of alkali metals with water is a fascinating and visually captivating chemistry demonstration.
30. Rainbow Flame
The rainbow flame experiment is a captivating and visually stunning chemistry demonstration that students should explore.
31. Sugar Yeast Experiment
This experiment not only introduces students to the concept of fermentation but also allows them to witness the effects of a living organism, yeast, on the sugar substrate.
32. The Thermite Reaction
The thermite reaction is a highly energetic and visually striking chemical reaction that students can explore with caution and under proper supervision.
This experiment showcases the principles of exothermic reactions, oxidation-reduction, and the high temperatures that can be achieved through chemical reactions.
33. Polishing Pennies
Polishing pennies is a simple and enjoyable chemistry experiment that allows students to explore the concepts of oxidation and cleaning methods.
34. Elephant Toothpaste
The elephant toothpaste experiment is a thrilling and visually captivating chemistry demonstration that students should try with caution and under the guidance of a knowledgeable instructor.
35. Magic Potion
Creating a magic potion is an exciting and imaginative activity that allows students to explore their creativity while learning about the principles of chemistry.
36. Color Changing Acid-Base Experiment

Through the color changing acid-base experiment, students can gain a deeper understanding of chemical reactions and the role of pH in our daily lives.
Learn more: Color Changing Acid-Base Experiment
37. Fill up a Balloon
Filling up a balloon is a simple and enjoyable physics experiment that demonstrates the properties of air pressure. By blowing air into a balloon, you can observe how the balloon expands and becomes inflated.
38. Jello and Vinegar

The combination of Jello and vinegar is a fascinating and tasty chemistry experiment that demonstrates the effects of acid on a gelatin-based substance.
Learn more: Jello and Vinegar
39. Vinegar and Steel Wool Reaction

This experiment not only provides a visual demonstration of the oxidation process but also introduces students to the concept of corrosion and the role of acids in accelerating the process.
Learn more: Vinegar and Steel Wool Reaction
40. Dancing Rice

The dancing rice experiment is a captivating and educational demonstration that showcases the principles of density and buoyancy.
By pouring a small amount of uncooked rice into a clear container filled with water, students can witness the rice grains moving and “dancing” in the water.
Learn more: Dancing Rice
41. Soil Testing Garden Science

Soil testing is a valuable and informative experiment that allows students to assess the composition and properties of soil.
By collecting soil samples from different locations and analyzing them, students can gain insights into the nutrient content, pH level, and texture of the soil.
Learn more: Soil Testing Garden Science
42. Heat Sensitive Color Changing Slime

Creating heat-sensitive color-changing slime is a captivating and playful chemistry experiment that students should try.
Learn more: Left Brain Craft Brain
43. Experimenting with Viscosity

Experimenting with viscosity is an engaging and hands-on activity that allows students to explore the flow properties of liquids.
Viscosity refers to a liquid’s resistance to flow, and this experiment enables students to investigate how different factors affect viscosity.
Learn more: Experimenting with Viscosity
44. Rock Candy Science

Rock candy science is a delightful and educational chemistry experiment that students should try. By growing their own rock candy crystals, students can learn about crystal formation and explore the principles of solubility and saturation.
Learn more: Rock Candy Science
45. Baking Soda vs Baking Powder

Baking soda and baking powder have distinct properties that influence the leavening process in different ways.
This hands-on experiment provides a practical understanding of how these ingredients interact with acids and moisture to create carbon dioxide gas.
46. Endothermic and Exothermic Reactions Experiment

The endothermic and exothermic reactions experiment is an exciting and informative chemistry exploration that students should try.
By observing and comparing the heat changes in different reactions, students can gain a deeper understanding of energy transfer and the concepts of endothermic and exothermic processes.
Learn more: Education.com
47. Diaper Chemistry

By dissecting a diaper and examining its components, students can uncover the chemical processes that make diapers so effective at absorbing and retaining liquids.
Learn more: Diaper Chemistry
48. Candle Chemical Reaction
The “Flame out” experiment is an intriguing and educational chemistry demonstration that students should try. By exploring the effects of a chemical reaction on a burning candle, students can witness the captivating moment when the flame is extinguished.
49. Make Curds and Whey

This experiment not only introduces students to the concept of acid-base reactions but also offers an opportunity to explore the science behind cheese-making.
Learn more: Tinkerlab
50. Grow Crystals Overnight

By creating a supersaturated solution using substances like epsom salt, sugar, or borax, students can observe the fascinating process of crystal growth. This experiment allows students to explore the principles of solubility, saturation, and nucleation.
Learn more: Grow Crystals Overnight
51. Measure Electrolytes in Sports Drinks
The “Measure Electrolytes in Sports Drinks” experiment is an informative and practical chemistry activity that students should try.
By using simple tools like a multimeter or conductivity probe, students can measure the electrical conductivity of different sports drinks to determine their electrolyte content.
52. Oxygen and Fire Experiment
The oxygen and fire experiment is a captivating and educational chemistry demonstration that students should try. By observing the effects of oxygen on a controlled fire, students can witness the essential role of oxygen in supporting combustion.
53. Electrolysis Of Water
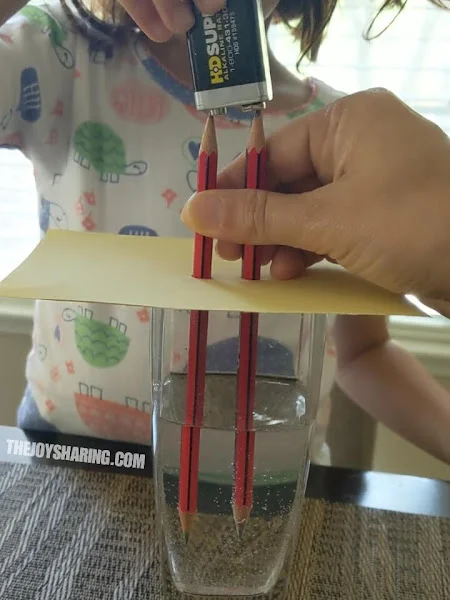
The electrolysis of water experiment is a captivating and educational chemistry demonstration that students should try.
Learn more: Electrolysis Of Water
54. Expanding Ivory Soap

The expanding Ivory Soap experiment is a fun and interactive chemistry activity that students should try. By placing a bar of Ivory soap in a microwave, students can witness the remarkable expansion of the soap as it heats up.
Learn more: Little Bins Little Hands
55. Glowing Fireworks

This experiment not only introduces students to the principles of pyrotechnics and combustion but also encourages observation, critical thinking, and an appreciation for the physics and chemistry behind.
Learn more: Glowing Fireworks
56. Colorful Polymer Chemistry

Colorful polymer chemistry is an exciting and vibrant experiment that students should try to explore polymers and colorants.
By combining different types of polymers with various colorants, such as food coloring or pigments, students can create a kaleidoscope of colors in their polymer creations.
Learn more: Colorful Polymer Chemistry
57. Sulfur Hexafluoride- Deep Voice Gas
This experiment provides a firsthand experience of how the density and composition of gases can influence sound transmission.
It encourages scientific curiosity, observation, and a sense of wonder as students witness the surprising transformation of their voices.
58. Liquid Nitrogen Ice Cream

Liquid nitrogen ice cream is a thrilling and delicious chemistry experiment that students should try. By combining cream, sugar, and flavorings with liquid nitrogen, students can create ice cream with a unique and creamy texture.
59. White Smoke Chemistry Demonstration

The White Smoke Chemistry Demonstration provides an engaging and visually captivating experience for students to explore chemical reactions and gases. By combining hydrochloric acid and ammonia solutions, students can witness the mesmerizing formation of white smoke.

60. Nitrogen Triiodide Chemistry Demonstration

The nitrogen triiodide chemistry demonstration is a remarkable and attention-grabbing experiment that students should try under the guidance of a knowledgeable instructor.
By reacting iodine crystals with concentrated ammonia, students can precipitate nitrogen triiodide (NI3), a highly sensitive compound.
61. Make a Plastic- Milk And Vinegar Reaction Experiment

Through the “Make a Plastic – Milk and Vinegar Reaction” experiment, students can gain a deeper understanding of the chemistry behind plastics, environmental sustainability, and the potential of biodegradable materials.
Learn more: Rookie Parenting
62. Eno and Water Experiment
This experiment not only introduces students to acid-base reactions but also engages their senses as they witness the visible and audible effects of the reaction.
63. The Eternal Kettle Experiment
By filling a kettle with alcohol and igniting it, students can investigate the behavior of the alcohol flame and its sustainability.
64. Coke and Chlorine Bombs
Engaging in this experiment allows students to experience the wonders of chemistry firsthand, making it an ideal choice to ignite their curiosity and passion for scientific exploration.
65. Set your Hand on Fire
This experiment showcases the fascinating nature of combustion and the science behind fire.
By carefully following proper procedures and safety guidelines, students can witness firsthand how the sanitizer’s high alcohol content interacts with an open flame, resulting in a brief but captivating display of controlled combustion.
66. Instant Ice Experiments
The Instant Ice Experiment offers an engaging and captivating opportunity for students to explore the wonders of chemistry and phase changes.
By using simple household ingredients, students can witness the fascinating phenomenon of rapid ice formation in just a matter of seconds.
67. Coke Cans in Acid and Base
Engaging in this experiment allows students to gain a deeper understanding of the chemical properties of substances and the importance of safety protocols in scientific investigations.
68. Color Changing Invisible Ink

The Color Changing Invisible Ink experiment offers an intriguing and fun opportunity for students to explore chemistry and learn about the concept of chemical reactions.
Learn more: Research Parent
Similar Posts:
- Top 100 Fine Motor Skills Activities for Toddlers and Preschoolers
- 37 Water Science Experiments: Fun & Easy
- Top 58 Creative Art Activities for Kids and Preschoolers
Leave a Comment Cancel reply
Save my name and email in this browser for the next time I comment.
- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information
Experiment - Chemical Bonding
College chemistry (chem 1010 ), metropolitan community college, nebraska, recommended for you, students also viewed.
- Activity - Post Lab - Classifying Matter
- Activity Sweet simulation of decay
- Activity - Classifying Matter Malone
- Background and Basic Structure of the Atom (1)
- Activity - Prelab Chemical Bonding and Vsepr Theory
- Activity - Prelab Counting by Weighing
Related documents
- Part two - using numbers in scientific measurements
- Experiment - Basic Concepts in Measurements
- Experiment - Prelab A Study of Matter and Properties Malone
- Experiment - Postlab Atoms and Light Malone
- Experiment - Postlab Density as a Property of Matter
- Chapter 11 and 13 Problem Set Answers
Preview text
Experiment chemical bonding, materials provided in the kit:, pictures of the crystal structures (provided online), aluminum foil weigh boats, two pieces of wire with alligator clips attached, materials provided by the student:, sucrose (sugar) - c 12 h 22 o 11, sodium chloride (table salt) – nacl, sodium bicarbonate (baking soda) – nahco 3, corn starch, two small squares of colored paper, plastic knife, dry cloth towel, distilled water, 9 volt battery, examining some properties of ionic and covalent compounds..
The following four experiments are designed to allow you to observe how sodium chloride (NaCl), an ionic compound, and sucrose (C 12 H 22 O 11 ), a covalent compound, are similar and how they differ.
Part 1. – visible structure of three compounds.
In the first part, you will be comparing the structure of the three compounds, two that are ionic (sodium chloride and sodium bicarbonate) and two that contain only covalent bonds (sugar and corn starch). Carefully examine each of the compounds using a magnifying glass and note any similarities and/or differences between the various compounds. Then compare the pictures of the compounds that were taken using a microscope to further compare their structures in classifying them as crystalline (regular repeating pattern or structure) or amorphous (no distinguishable pattern in structure formation). This pictures can be found in Laboratory 13 folder.
Compound Observation(s) directly on sample Observation(s) based on pictures
Sodium chloride, NaCl
White small cubes, grainy looking Small cubes that are dark on the edges and clear in the middle, crystal in appearance
Sucrose, C 12 H 22 O 11
White small circle crystals that are grainy looking
Clear cubes squares and cylinders
Sodium bicarbonate
Fluffy white powder Black tiny balls
Corn Starch
Cream colored powder, grainy in appearance
White small cloudy grains
Comparison of the electrostatic potential of NaCl and sucrose by determining whether or not they respond to static electricity.
Materials: Sucrose – C 12 H 22 O 11 Sodium chloride – NaCl Two small squares of colored paper A plastic knife A dry cloth towel
Procedure Observations 1. Place a sample of dry salt about the size of half an aspirin tablet on a flat piece of paper and spread it out to make a flat layer.
Small crystals that are almost see through
- Place a similar amount of dry granulated sugar on another flat piece of paper and spread it out to make a flat layer.
Small crystals that are cloudy in appearance
- Rub the plastic knife with a dry towel about 20 times to give it an electrostatic charge.
- Lower the knife so that it touches the center of the sample of salt. Record your observations.
The salt attached to the knife and the knife didn’t really make contact with the salt
- Recharge the plastic knife by again rubbing it with a dry towel about 20 times.
- Lower the knife so that it touches the center of the sugar. Record your observations.
- Take a picture of the compound interaction
Almost all of the sugar was attached to the knife
Part 3. Comparison of the melting point of an ionic versus a covalent compound.
Materials: Matches Sucrose Sodium chloride Two aluminum foil weigh boats A pair of tweezers
Procedure Observations 1. Place a sample of sugar about the size of half an aspirin tablet in one of the aluminum foil weigh boats. 2. Holding the aluminum foil weigh boat with the tweezers, light a match and hold it under the aluminum foil just below the sugar. Record your observations. 3. Set the sample aside to thoroughly cool before discarding in the indicated container. Note: if the sugar begins to burn and char, immediately remove the match. Take a picture of the result and attach to your report.
The sugar started to get larger and stuck to the bottom of the boat
- Place a sample of salt about the size of half an aspirin tablet (or the same amount as the sugar) in the second aluminum foil weigh boat.
- Holding the aluminum foil weigh boat with the tweezers, light a match and hold it under the aluminum foil just below the salt. Record your observations.
- Set the sample aside to thoroughly cool before discarding in the indicated container. Note: if the salt begins to burn and char, remove the match. Take a picture of the result and attach to your report.
The salt got larger and stuck to the bottom of the boat
Part 4. To determine if a solution of sodium chloride and/or sucrose will conduct electricity.
Materials Granulated sucrose Sodium chloride Distilled water
Two sheets of colored paper Two pieces of wire with alligator clips attached A 9-volt battery Procedure
- Use the two pieces of paper to make paper dishes by folding each into quarters and crimping the edges to form the dish.
- Using the alligator clips, attach one wire to each of the electrodes on the 9-volt battery. Make sure that the ends of the wire are not touching each other. These wires are now the electrodes.
- Place the battery with the wires attached in a 30 mL or 50 mL beaker for support. Take a picture of your set up and attach to your report. Observations
- Place a sample of salt about the size of an aspirin tablet in the middle of one paper dish.
- Add a small amount of water on top of the salt. Make sure that you add enough water to dissolve some salt. Record its appearance on the right.
The salt is no longer white and crystal in appearance, but rather round and semi dissolved in the water
- Test the resulting salt solution with your conductivity/electrolysis apparatus. Hold one wire in each hand and dip the electrodes into the solution. Make sure that the ends of the wires are submerged in the solution but are NOT touching each other. Look closely at the wire tip of each electrode and record your observations. Take a picture of the result and attach to your report.
The crystals became bigger and round, spreading throughout the water
- Rinse the ends of the wire with distilled water to remove any residue.
- Place a sample of sucrose (sugar) about the size of an aspirin tablet in the middle of the second paper dish.
- Add a small amount of water on top of the sucrose. Make sure that you add enough water to dissolve some salt. Record its appearance on the right.
The crystals became bigger and round spreading throughout the water
- Test the resulting sucrose solution with your conductivity/electrolysis apparatus. Hold one wire in each hand and dip the electrodes into the solution. Make sure that the ends of the wires are submerged in the solution but are NOT touching each other. Look closely at the wire tip of each electrode and record your observations. Take a picture of the result and attach to your report.
Nothing was visible, the water absorbed into the paper
- Multiple Choice
Course : College Chemistry (CHEM 1010 )
University : metropolitan community college, nebraska.

- More from: College Chemistry CHEM 1010 Metropolitan Community College, Nebraska 80 Documents Go to course
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
Teaching Expertise
- Classroom Ideas
- Teacher’s Life
- Deals & Shopping
- Privacy Policy
18 Engaging Chemical Bonding Activities for Your Classroom
June 2, 2023 // by Lauren Du Plessis
We’ve got 18 creative chemical bonding activities that will help you make the concept come alive in your classroom! These activities are designed to spark curiosity, promote teamwork, and deepen students’ understanding of chemical bonding. Allow us to guide you through the wonderful world of atoms and molecules!
1. Bonding Bingo
Play a fun game of Bingo but with a twist! Instead of numbers, the Bingo cards can feature different types of chemical bonds. Students will need to identify the bonding types as they mark their cards; reinforcing their knowledge in an interactive way!
Learn More: Bingo Baker
2. Molecular Modeling
Provide students with molecular model kits and challenge them to build various compounds. In doing so, they’ll gain a hands-on understanding of how atoms come together to form different types of bonds.
Learn More: Edu RSC
3. Bonding Puzzles
Create puzzles where students will need to match molecular structures with the correct bonding types. This activity will test their knowledge and critical thinking skills while making learning more enjoyable.
Learn More: Teachers Pay Teachers
4. Bonding Art
Encourage students to express their understanding of chemical bonding through art. They can create colorful illustrations or paintings that depict different bonding scenarios.
Learn More: U Waterloo
5. Bonding Stations
This activity promotes exploration and deeper understanding. Set up different stations around the classroom; each representing a specific bonding type. Students must then rotate through the stations to observe and discuss the characteristics of each bond.
6. Bonding Charades
Put a fun twist on learning by playing a game of charades! Have your students form groups of 6-8 learners, plan their charade skit, and then perform it for their classmates to make guesses as to what bonds they’re acting out.
Learn More: Slide Player
7. Bonding Relay Race
Organize a relay race where students will need to answer bonding-related questions before passing the baton to the next team member. This activity fosters teamwork and quick thinking while reviewing bonding concepts; making it a great activity to use when wrapping up the unit.
8. Bonding Scavenger Hunt
Hide clues related to chemical bonding around the classroom or school. Have your learners work together to solve the clues to find different bonding concepts.
9. Bonding Role Play
Add a theatrical element to your lesson with fun role-play! Assign your students roles as atoms and have them act out the formation of chemical bonds. They can interact, bond, and break apart to demonstrate different bonding types.
10. Bonding Debate
Divide your class into teams and assign the groups different perspectives on a controversial bonding topic. Students must then engage in a debate; defending their viewpoints and analyzing the strengths and weaknesses of various bonding theories.
Learn More: Clarku Edu
11. Bonding Comic Strip
Here’s an activity for learners who are artistically inclined! Have your students create comic strips that illustrate different bonding scenarios. They can use speech bubbles and illustrations to showcase their understanding in a visually engaging format.
Learn More: Studocu
12. Bonding Reflection Journals
Encourage students to keep reflection journals where they can record their thoughts and observations about bonding concepts throughout the unit.
Learn More: Marcelo Villarreal01
13. Bonding Field Trip
Take your class on a field trip to a local science museum or university chemistry department. There, students will have the opportunity to see real-life examples of chemical bonding in action and interact with experts in the field!
Learn More: Mr. Bond Science Guy
14. Bonding Battleship
Adapt the classic game of Battleship by using molecular structures as the ships. Students can take turns guessing coordinates and identifying the type of bonding in the targeted molecule.
Learn More: PUBS
15. Bonding Tic-Tac-Toe
Create a tic-tac-toe board where each square contains a chemical bonding concept. Students will have to take turns answering questions or providing examples related to the concepts to then mark their X or O. What a strategic and engaging way to reinforce their knowledge!
16. Bonding Relay Diagrams
This activity promotes communication and teamwork while helping your students review their knowledge of bonding concepts. Divide your class into 2 teams and provide them with diagrams of different bonding types. One member from each team starts the relay by explaining their diagram to the next teammate, who then passes on the information until the final teammate completes the diagram.
17. Bonding Kahoot
Create a Kahoot quiz with questions about chemical bonding. Students can either compete individually or in teams; answering questions in a fun and interactive way.
Learn More: AZ Chemistry
18. Bonding Video Project
Assign groups of students to create short videos explaining different bonding concepts. They can use animations, demonstrations, or skits to convey their understanding. This activity allows for creativity and provides an opportunity for students to showcase their knowledge.
Learn More: RicochetScience

Teacher Resource Center
Pasco partnerships.

2024 Catalogs & Brochures
Types of bonding.
Students use a Conductivity Sensor to predict the type(s) of bonding between atoms in solutions made with different substances.
Supports NGSS Performance Expectation HS-PS1-3: Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles.
Grade Level: High School
Subject: Chemistry
Student Files
Teacher Files
Login to PASCO Portal to access teacher files and sample data.
Watch Video

Use a Conductivity Sensor to predict the type(s) of bonding between atoms in solutions made with different substances. Supports PASCO's Chemistry Starter Lab...
Featured Equipment

OHAUS Scout SKX Balance 220g
Combines range, resolution and low cost, making it ideal for the student science lab. 220 g version.

Wireless Conductivity Sensor with Display
This water resistant sensor connects via Bluetooth® to measure both conductivity (ionic content in solution) and total dissolved solids in solution, and features a bright display of real-time readings for convenience in a variety of labs.

Wireless Conductivity Sensor
This waterproof sensor connects via Bluetooth® to measure both conductivity (ionic content in solution) and total dissolved solids.

Essential Chemistry Starter Lab Kit
This Starter Lab Station includes the wireless temperature, conductivity, pressure, and pH sensors to perform key lab activities from the Essential Chemistry Student Lab Manual.
Many lab activities can be conducted with our Wireless , PASPORT , or even ScienceWorkshop sensors and equipment. For assistance with substituting compatible instruments, contact PASCO Technical Support . We're here to help. Copyright © 2018 PASCO
Source Collection: Lab #05
Source Collection: Lab #34
Essential Chemistry Teacher Lab Manual
More experiments.
Advanced Placement
- Energy In Chemical Reactions
High School
- Research Enhancement: Airbags and Consumers
- Determining Limiting Reactants
- Fragrant Esters
- Le Chatelier's Principle
- Varying Reaction Rates
- Science, Tech, Math ›
- Chemistry ›
- Projects & Experiments ›
10 Cool Chemistry Experiments
ThoughtCo / Hilary Allison
- Projects & Experiments
- Chemical Laws
- Periodic Table
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Chemistry is king when it comes to making science cool. There are many interesting and fun projects to try, but these 10 chemistry experiments might be the coolest.
Whether you want to witness color transformations with copper and nitric acid or create a foam spectacle with hydrogen peroxide and potassium iodide, there's something here to spark curiosity in everyone. There's even a famous chemical reaction that will emit blue light and a characteristic barking or woofing sound.
Copper and Nitric Acid
When you place a piece of copper in nitric acid , the Cu 2+ ions and nitrate ions coordinate to color the solution green and then brownish-green. If you dilute the solution, water displaces nitrate ions around the copper, and the solution changes to blue.
Hydrogen Peroxide with Potassium Iodide
Affectionately known as elephant toothpaste , the chemical reaction between peroxide and potassium iodide shoots out a column of foam. If you add food coloring, you can customize the "toothpaste" for holiday-colored themes.
Any Alkali Metal in Water
Any of the alkali metals will react vigorously in water . How vigorously? Sodium burns bright yellow. Potassium burns violet. Lithium burns red. Cesium explodes. Experiment by moving down the alkali metals group of the periodic table.
Thermite Reaction
The thermite reaction essentially shows what would happen if iron rusted instantly, rather than over time. In other words, it's making metal burn. If the conditions are right, just about any metal will burn. However, the reaction usually is performed by reacting iron oxide with aluminum:
Fe 2 O 3 + 2Al → 2Fe + Al 2 O 3 + heat and light
If you want a truly stunning display, try placing the mixture inside a block of dry ice and then lighting the mixture.
Coloring Fire
SEAN GLADWELL / Getty Images
When ions are heated in a flame, electrons become excited and then drop to a lower energy state, emitting photons. The energy of the photons is characteristic of the chemical and corresponds to specific flame colors . It's the basis for the flame test in analytical chemistry , plus it's fun to experiment with different chemicals to see what colors they produce in a fire.
Make Polymer Bouncy Balls
Who doesn't enjoy playing with bouncy balls ? The chemical reaction used to make the balls makes a terrific experiment because you can alter the properties of the balls by changing the ratio of the ingredients.
Make a Lichtenberg Figure
A Lichtenberg figure or "electrical tree" is a record of the path taken by electrons during an electrostatic discharge. It's basically frozen lightning. There are several ways you can make an electrical tree.
Experiment with 'Hot Ice'
Hot ice is a name given to sodium acetate, a chemical you can make by reacting vinegar and baking soda. A solution of sodium acetate can be supercooled so that it will crystallize on command. Heat is evolved when the crystals form, so although it resembles water ice, it's hot.
Barking Dog Experiment
The Barking Dog is the name given to a chemiluminescent reaction involving the exothermic combination of either nitrous oxide or nitrogen monoxide with carbon disulfide. The reaction proceeds down a tube, emitting blue light and a characteristic "woof" sound.
Another version of the demonstration involves coating the inside of a clear jug with alcohol and igniting the vapor. The flame front proceeds down the bottle , which also barks.
Dehydration of Sugar
When you react sugar with sulfuric acid , the sugar is violently dehydrated. The result is a growing column of carbon black, heat, and the overwhelming odor of burnt caramel.
Easy Science Experiments
Want something less extravagant but still fun? These easy science experiments are doable with items you likely already have at home—from creating invisible ink with baking soda to making homemade ice cream in a plastic bag.
- Where to Buy Saltpeter or Potassium Nitrate
- Vitamin C Determination by Iodine Titration
- Cool Dry Ice Experiments
- How to Make Bubbles That Don't Pop
- Equation for the Reaction Between Baking Soda and Vinegar
- 5 Ways to Make Glue
- How to Grow Table Salt or Sodium Chloride Crystals
- Color Change Chemistry Experiments
- How to Melt Aluminum Cans at Home
- 10 Cool Chemistry Demonstrations for Educators
- Science Projects for Every Subject
- 10 Fun Chemistry Demonstrations and Experiments
- Valentine's Day Chemistry
- Thames & Kosmos Chem 3000 Chemistry Kit Review
- Halloween Reaction or Old Nassau Reaction
- How to Do the Color Change Chameleon Chemistry Demonstration
- WordPress.org
- Documentation
- Learn WordPress
- Members Newsfeed
18 Engaging Chemical Bonding Activities for Your Classroom
- Art Education

Introduction:
Chemical bonding is a fundamental concept in chemistry, playing a vital role in the formation of different substances. Teaching students about chemical bonds can be challenging, but engaging activities can make learning about this topic both fun and informative. Here are 18 exciting chemical bonding activities that will help your students grasp this complex subject while having a great time.
1. Molecular Models:
Have students create models of molecules using marshmallows, gumdrops, or playdough as atoms and toothpicks as bonds. This hands-on activity provides a visual and tactile representation of different types of chemical bonds.
2. Building Molecules with Chemical Formula Cards:
Using cards with printed chemical formulae, challenge students to build corresponding molecular models using available materials like Lego blocks or molecular model kits.
3. Edible Chemistry:
Allow students to explore the world of chemical bonding through edible experiments! Create simple recipes that include bond formation, such as making rock candy or chocolate fondue.
4. Online Bonding Games:
Explore online games and quizzes that focus on chemical bonding concepts. These interactive experiences can reinforce understanding while making learning enjoyable.
5. Chemical Bond Scavenger Hunt:
Organize a scavenger hunt where students search for items within the classroom that represents various types of chemical bonds and discuss why those items exemplify those bonds.
6. Crafting Ionic Compounds:
Let your students get creative by making ionic compound structures out of clay, pipe cleaners, or beads labeled as cations and anions.
7. Poster Competition:
Split the class into groups to create posters illustrating different types of chemical bonds (ionic, covalent, metallic) and their properties. Have a friendly competition by hanging up completed posters for everyone to see and vote on their favorite.
8. “Speed Dating” Bond Formation:
Students “speed date” with one another to find suitable bond partners based on valence electrons. This can be a fun and memorable way to demonstrate atomic bonding.
9. Atomic Bond Puzzles:
Create puzzle pieces from cardboard or cardstock with different atoms on them. Encourage students to fit the puzzle pieces together to form bonded molecules.
10. Guess the Bond Game:
Place images of various molecules on the board and have students guess the type of chemical bond that holds them together.
11. Gumdrop Molecules Lab:
Students build molecules using gumdrops and toothpicks to investigate molecular geometry and bond angles.
12. Chemical Bond Role-Playing:
Have students role-play as atoms with varying electron configurations, while interacting with one another to form chemical bonds.
13. Balloon Simulations:
Simulate bond polarity by inflating balloons with different gases (air, helium, or nitrogen) and observing the resulting behavior when brought into proximity.
14. 3D Printing Activity:
Design and print 3D molecular models for a hands-on exploration of chemical bonding structure.
15. The Art of Chemical Bonding:
Invite students to create artistic representations of various chemical bonding concepts, like mixed-media collages, paintings, or sculptures.
16. Classroom Debate:
Organize debates around topics related to chemical bonding, such as covalent vs. ionic bonds or electronegativity’s role in bond formation.
17. Molecular Dance-Off:
Choreograph a dance routine based upon different types of chemical bonds and their attributes, incorporating both movement and rhythm to represent each bond variety creatively.
18. Chemical Bond Skits:
Assign groups of students specific chemical bonding scenarios to perform as skits in front of their peers, emphasizing the significance of each reaction presented.
Conclusion:
Chemical bonding is an essential component in understanding chemistry, but it doesn’t have to be a dull topic. By incorporating these engaging
Related Articles

Creating an engaging and informative bulletin board in your classroom doesn't have…

Crafting with children can be a fun and educational experience that stimulates…

As spring blossoms, it's time to get the little ones outdoors for…

Pedagogue is a social media network where educators can learn and grow. It's a safe space where they can share advice, strategies, tools, hacks, resources, etc., and work together to improve their teaching skills and the academic performance of the students in their charge.
If you want to collaborate with educators from around the globe, facilitate remote learning, etc., sign up for a free account today and start making connections.
Pedagogue is Free Now, and Free Forever!
- New? Start Here
- Frequently Asked Questions
- Privacy Policy
- Terms of Service
Are you sure you want to delete post?
This post cannot be restored anymore.
- Registration
Don't you have an account? Register Now! it's really simple and you can start enjoying all the benefits!
We just sent you an Email. Please Open it up to activate your account.
I allow this website to collect and store submitted data.
Projects On Chemical Bonding
Chemical bonds hold together atoms in compounds. There are two kinds of chemical bonds: covalent and ionic bonds. Covalent bonds form when two atoms share electrons to fill their outermost valence shells. Ionic bonds form when one atom steals the electrons from another atom, creating positive and negative ions binding the two atoms together. Chemical bonding projects can help students understand these difficult and elusive concepts.
Element Cards
Create element cards with different elements that will create covalent and ionic bonds. All elements should have their outer valence electron shells shown. A common compound that has a covalent bond is sodium (Na) and chlorine (Cl). Sodium has one valence electron, and chlorine has seven. By sharing sodium's electron, both elements are able to have a complete outer shell. A common ionic bond is hydrogen (H) and chlorine. Hydrogen has one outer valence electron, just like sodium; however, hydrogen only has one electron. Chlorine takes hydrogen's electron. It is not sharing. Repeat with other cards, creating different covalent and ionic bonds.
Heat Testing
Covalent and ionic bonds have different bonding strengths. Adding energy–heat in most cases–will show those differences within just a few minutes. Take a known covalently bonded compound and heat it. The compound should melt after only a few minutes. Provide an ionic compound and heat it. The ionic bond should not break under the heat provided in a lab setting.
Dissolving Test
Another difference between covalent and ionic bonds is their ability to dissolve. Use both water and ethanol. Dissolve compounds in both liquids. Both covalent and ionic compounds will dissolve in water. However, in ethanol only covalent bonded compounds will dissolve. Ionic compounds will not dissolve in ethanol.
Conduction of Electricity
Once the compounds have been dissolved in water, they can be tested to see whether they convey electricity. Covalent bonds will not convey electricity. Dissolved ionic compounds will convey electricity.
- Science Spot: Bonding Basics 2010
Cite This Article
Carpenter, Michael E. "Projects On Chemical Bonding" sciencing.com , https://www.sciencing.com/projects-chemical-bonding-7513341/. 24 April 2017.
Carpenter, Michael E. (2017, April 24). Projects On Chemical Bonding. sciencing.com . Retrieved from https://www.sciencing.com/projects-chemical-bonding-7513341/
Carpenter, Michael E. Projects On Chemical Bonding last modified August 30, 2022. https://www.sciencing.com/projects-chemical-bonding-7513341/
Recommended

COMMENTS
In this experiment you will be able to see a chemical bond. Dye made from kool aid and vinegar will make a bond, or “stick” to the fabric of a cotton t-shirt – kool aid tie dye! Some chemical bonds are strong and the two substances really stick to each other. Some chemical bonds are weak.
These experiments provide hands-on experiences illuminating the intricacies of chemical reactions, molecular structures, and elemental properties. 1. Covalent Bonds.
The following four experiments are designed to allow you to observe how sodium chloride (NaCl), an ionic compound, and sucrose (C 12 H 22 O 11 ), a covalent compound, are similar and how they differ. Part 1. – visible structure of three compounds.
In the first part of this lab you will investigate how ionically bonded and covalently bonded substances behave differently in their conduction of electricity. You will do this by using a simple anodizing apparatus. A stainless steel screw and an iron nail will be used for the electrodes.
Use the short sticks for single bonds. Use two long flexible sticks for a double bond and three long flexible sticks for a triple bond. Compare each model constructed to the molecular shapes described in the Theory section. Then identify and record its correct shape name and its bond angles.
We’ve got 18 creative chemical bonding activities that will help you make the concept come alive in your classroom! These activities are designed to spark curiosity, promote teamwork, and deepen students’ understanding of chemical bonding.
Types of Bonding. Use a Conductivity Sensor to predict the type(s) of bonding between atoms in solutions made with different substances. Supports PASCO's Chemistry Starter Lab...
There are many interesting and fun projects to try, but these 10 chemistry experiments might be the coolest. Whether you want to witness color transformations with copper and nitric acid or create a foam spectacle with hydrogen peroxide and potassium iodide, there's something here to spark curiosity in everyone.
Here are 18 exciting chemical bonding activities that will help your students grasp this complex subject while having a great time. 1. Molecular Models: Have students create models of molecules using marshmallows, gumdrops, or playdough as atoms and toothpicks as bonds.
Chemical bonding projects can help students understand these difficult and elusive concepts. Chemical bonds hold together atoms in compounds. There are two kinds of chemical bonds: covalent and ionic bonds.