Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- Science Education Policy Alliance
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items

Separating sand and salt by filtering and evaporation
In association with Nuffield Foundation
- Four out of five
Task students to separate an insoluble material from a soluble one in this experiment using sand and salt
This is a very straightforward experiment. It can be carried out individually or in groups of two. Pupils must stand up during heating activities and beware of hot salt spitting when evaporation is almost complete.
- Eye protection
- Beaker, 250 cm 3
- Glass stirring rod
- Filter funnel
- Filter paper
- Conical flask, 250 cm 3
- Evaporating basin
- Bunsen burner
- Heat resistant mat
- Mixture of sand and sodium chloride (salt), about 6–7 g per group of students (a suitable sand–salt mixture should contain approximately 20% salt by mass)
Health, safety and technical notes
- Wear eye protection throughout this experiment.
- Pupils must stand up during heating activities and beware of hot salt spitting when evaporation is almost complete.
- Sodium chloride (eg table salt), NaCl(s) - see CLEAPSS Hazcard HC047b .
- Pour the sand–salt mixture into the beaker so that it just covers the base.
- Add about 50 cm 3 of water, or add water until the beaker is about one-fifth full.
- Stir the mixture gently for a few minutes.
- Filter the mixture into a conical flask.
- Pour the filtrate into an evaporating basin.
- Heat the salt solution gently until it starts to decrepitate (spit). CARE: Keep eye protection on and do not get too close.
- Turn off the Bunsen burner and let the damp salt dry in the dish.
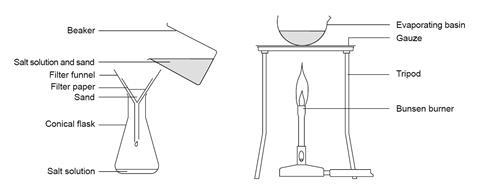
Source: Royal Society of Chemistry
Equipment for a class experiment to separate a mixture of sand and salt.
Teaching notes
If desired, the experiment can be extended to isolate dry samples of sand and salt. To do this, the damp sand in the filter paper can be transferred to another sheet of dry filter paper, and, by folding and dabbing, the sample can be dried. If necessary, another piece of filter paper can be used.
Students often like to present their specimens in small bottles for approval, so a spatula could be used to accomplish this. While the first student of a pair is transferring the sand, the other can be scraping the dried salt from the evaporating dish and transferring it to another specimen bottle.
If this extension is carried out, the students should be encouraged to label the bottles. They should be told that all samples prepared in this way need to be labelled, even if in this case, it should be obvious which substance is which.
Student questions
- Why can sand and salt be separated using this experiment?
- Why is the salt, sand and water mixture stirred in step 3?
- Why is the salt solution heated in step 6?
- How might the final traces of water be removed from your samples to ensure that they are totally dry?
- Give two reasons why the sand you have obtained might still be contaminated with salt.
- How could you adapt your experiment to obtain a purer sample of sand?
- Give two reasons why the salt you have obtained might still be contaminated with sand.
- How could you adapt your experiment to obtain a purer sample of salt?
Primary science teaching notes
If you teach primary science, the following information is designed to help you use this resource.
Skill development
Children will develop their working scientifically skills by:
- Drawing conclusions and raising further questions that could be investigated, based on their data and observations.
- Using appropriate scientific language and ideas to explain, evaluate and communicate their methods and findings.
Learning outcomes
Children will:
- Observe that some materials will dissolve in liquid to form a solution.
- Describe how to recover a substance from a solution.
- Use knowledge of solids, liquids and gases to decide how mixtures might be separated, including through filtering, sieving and evaporating.
- Demonstrate that dissolving, mixing and changes of state are reversible changes.
Concepts supported
Children will learn:
- That there are various techniques that can be used to separate different mixtures.
- That dissolving is a reversible reaction.
- That not all solids are soluble.
- That the rate of dissolving can be affected by various factors.
- That melting and dissolving are not the same process.
Suggested activity use
This activity can be used as a whole-class investigation, with children working in small groups or pairs to look at how to separate the salt and sand. This could provide a stimulus for further investigations looking at how to separate other mixtures of solids, either of different particle sizes or by solubility.
Practical considerations
Primary schools often don’t have Bunsen burners, so viable alternatives need to be sourced. Similarly, it may be difficult to source the equipment needed to evaporate water to recover the dissolved salt. Head stands and tea lights can work well as possible alternatives.
When carrying out this activity be aware that some insoluble solids are able to form suspensions. This is where the particles appear to have dissolved, when in fact they have been spread out throughout the liquid. A good indicator that a suspension has formed is that the liquid will go cloudy or the particles can be heard scraping as the mixture is stirred.
The layout of this activity is very prescriptive as the procedure is set out on a step by step basis. An open challenge activity, with children working in small groups and devising their own methods, would extend the children’s thinking. Different groups’ suggestions could be compared and evaluated as a class.
Additional information
This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology .
© Nuffield Foundation and the Royal Society of Chemistry
- 11-14 years
- 14-16 years
- Practical experiments
- Compounds and mixtures
Specification
- AT.4 Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation.
- Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. These physical processes do not involve chemical reactions and no new substances are made.
- AT4 Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation.
- 4 Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation
- Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation
- (i) atoms/molecules in mixtures not being chemically joined and mixtures being easily separated by physical processes such as filtration, evaporation, chromatography and distillation
- 1.9.5 investigate practically how mixtures can be separated using filtration, crystallisation, paper chromatography, simple distillation or fractional distillation (including using fractional distillation in the laboratory to separate miscible liquids…
- 2. Develop and use models to describe the nature of matter; demonstrate how they provide a simple way to to account for the conservation of mass, changes of state, physical change, chemical change, mixtures, and their separation.
Related articles

Ionic bonding | Developing understanding | 14–16 years
By Helen Harden and Neil Goalby
Develop learners’ understanding of ionic lattices through comparing 2D and 3D representations

Popping good chemistry
Investigate simple chemical reactions, irreversible changes and gases around us with this exciting experiment

Solubility | Review my learning worksheets | 14–16 years
By Lyn Nicholls
Identify learning gaps and misconceptions with this set of worksheets offering three levels of support
2 readers' comments
Only registered users can comment on this article., more experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet


Practical potions microscale | 11–14 years
By Kirsty Patterson Four out of five
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud

Charcoal Water Purifying Experiment
Sharing is caring!

Do you use some sort of charcoal water filter in your home? You might have a pitcher with a charcoal filter r or a filter that attaches to your faucet . Have you ever wondered how that process actually works? Try this charcoal water purifying experiment to learn all about it!
Science Experiments For Independent Learning
From start to finish, she is responsible for the experiment. Of course, she can ask questions or get help if she needs it, but I have found that the more she does the experiments, the less help she needs.
During the experiment, my daughter keeps notes on an experiment sheet. The rest of this blog post is her lab report as recorded on her experiment worksheet.
This charcoal water purifying experiment is a great experiment for middle school students to try on their own.
Can Charcoal Remove Molecules From Water?

My pre-experiment hypothesis is yes, charcoal can remove molecules from water. This is based on my knowledge of using charcoal filters to clean water.
- a measuring cup
- 2 baby food jars with lids
- red food coloring
Fill a measuring cup with 1/2 cup of water. Add 8 drops of red food coloring to the water and stir until mixed. Now, add 1/4 cup of colored water to each of the baby food jars.
To one jar, add 2 teaspoons of the activated carbon. Put the lids on both jars and set the jars in a place where they won’t be disturbed for a couple days.
Note the color of the jars when the carbon was added, 4 hours later, 24 hours later, and each day for 3 days.
Observations and Results
At the beginning of the experiment, the color of the water in the jars was the same.

By the next day, the water was almost totally clear in the activated carbon jar and the other jar was just as red as the beginning. On the second day after the experiment began, the water in the activated carbon jar was totally clear.
My results conclude that my hypothesis was correct. Charcoal can remove molecules from the water.
What Happened?
Activated charcoal is charcoal that has been specially treated to create cracks and holes in the charcoal. This treatment process is called activation and involves heating the charcoal to a high temperature. The high temperature changes the structure of the charcoal and makes it more porous and causes cracking. The cracking creates a greater surface area and more bonding sites. It is these binding sites that attract many chemicals in liquid and chemical form.
The food coloring is made of molecules. These molecules are bigger than water molecules.
When the food coloring, water, and charcoal are mixed together, the food coloring molecules break their bond to the water and attach to the carbon molecules of the activated carbon.
When the food coloring molecules (a liquid) “unstick” from the water (another liquid) and attach to the activated carbon (a solid), this is called adsorption. Adsorption happens when a liquid molecule separates from another liquid molecule and attaches to the molecule of a solid.
This is how the activated charcoal is able to remove the food coloring from the water. The food coloring molecules bind with the charcoal and come out of the water solution. This property of activated charcoal is what makes it a great filter/purifier.
Try this at home with your kids or let them try it for themselves!
For More Chemistry Activities
Check out The Homeschool Scientist’s Chemistry Resource Page!
Check out our printable unit on The Periodic Table of the Elements
Studying the properties of water? Check out these activities that demonstrate polarity, adhesion, cohesion, and more .

I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School
Homemade Water Filter Experiment
July 6, 2019 By Emma Vanstone 11 Comments
Today we are looking at filtering and how it can be used to clean dirty water. This water filter science project is very simplified but gives kids a great overview of how water purification is carried out to give us lovely clean drinking water.
Do not drink the water in the activity

Water Filter Science Project
Filtering water – what you need.
Dirty water ( or mud and clean water )
Coffee filter/paper towel/muslin
An empty bottle or other containers
Filtering Water Experiment
The great thing about this experiment is that you can design it however you like.
We set up coffee filters containing sand, stones and then just plain filters.

We carefully poured the same amount of water through each filter and observed the results.

More ideas to try – Water Filter Science Project
Set up an experiment where the conditions are:
1 coffee filter
2 coffee filters
3 coffee filters
Is kitchen roll better than a coffee filter, would just a sieve work?
You could also work in stages, so try a colander, then a sieve and then a paper towel. Each stage should trap smaller and smaller particles.
How do filters work?
A filter is a porous material which a liquid can be passed though to separate the liquid from solids suspended in it.
More Filtering Investigations
Make a toy filter .
Try filtering potions using a sieve and colander.
Can you filter water using sand and stones ?

More Science for Kids
I’ve got a fun collection of water science experiments , including a water cycle activity, dissolving experiment, ice investigation and density trick!
Try one of our easy science experiments for kids to do at home ! We’ve got egg experiments, ice experiments, paper helicopters, STEM challenges and lots more science fun for kids of all ages!

Suitable for Key stage 2 Science
Properties and Changes of Materials
Use knowledge of solids, liquids and gases to decide how mixtures might be separated, including through filtering, sieving and evaporating.

Last Updated on August 6, 2024 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
May 15, 2012 at 11:49 pm
This is such a neat experiment. I bet it would be fun to let the kids think of better ways to filter the water. It will definitely give them a better appreciation for our clean water!
May 18, 2012 at 8:22 pm
So simple, and yet so fun! A very good way to learn about filters and clean water. We will definitely have to try this one, thanks 🙂
May 22, 2012 at 8:21 pm
We did that once too and it’s amazing how much gets filtered out and how gross that water still is. I think yours was more easily visble, we have very clay like dirt, so it didn’t quite dissolve right. Thanks for linking up to Science Sunday!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

IMAGES
COMMENTS
Filtration is one important step in water cleanup. During the filtration process, particles or impurities such as chemicals and bacteria are separated from the solution that is filtered. The method of separation can be mechanical, physical, chemical, or even biological.
Teaching notes. If desired, the experiment can be extended to isolate dry samples of sand and salt. To do this, the damp sand in the filter paper can be transferred to another sheet of dry filter paper, and, by folding and dabbing, the sample can be dried.
CAUTION: Adult supervision is required when handling chemicals and/or cutting objects.Remember to use proper safety equipment when experimenting. Because we won't disinfect our water sample for this water project and will just be making an example water filtration system, it is NOT safe to drink as this purification process and treatment process we will experiment with will not provide safe ...
Filtration Techniques Revised 8/6/12 4 hose is part of the filtration system and should be heavy enough to prevent pinching or collapse under external atmospheric pressure. To filter a sample, turn on the aspirator and carry out the filtration in the same manner described for gravity filtration. (Note: NEVER pry
Science Experiments For Independent Learning. My seventh grader is becoming more and more of an independent learner. One of the ways we have fostered this is through science experiments like this one. She was given several good e xperiment books and told to choose an experiment and do it. From start to finish, she is responsible for the experiment.
Jul 6, 2019 · Water Filter Science Project Filtering Water – What you need. Dirty water ( or mud and clean water ) Funnels. Coffee filter/paper towel/muslin. Sand. Stones. An empty bottle or other containers. Filtering Water Experiment. The great thing about this experiment is that you can design it however you like.